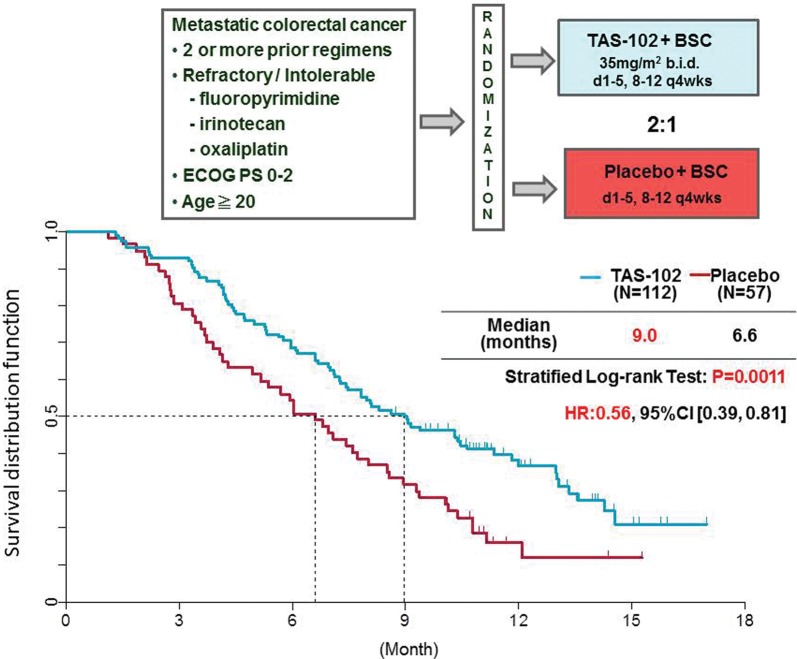
Figure 7.
Result of the PII trial of TAS-102 in third/fourth line colorectal cancer. Patients were randomly assigned in a 2:1 ratio to either TAS-102 plus best supportive care (BSC) or placebo plus BSC. A dose of 35 mg/m2 TAS-102 was taken orally twice a day. TAS-102 or placebo was taken in a 28-day cycle: a 2-week cycle of 5 days of treatment followed by a 2-day rest, and then a 14-day rest.