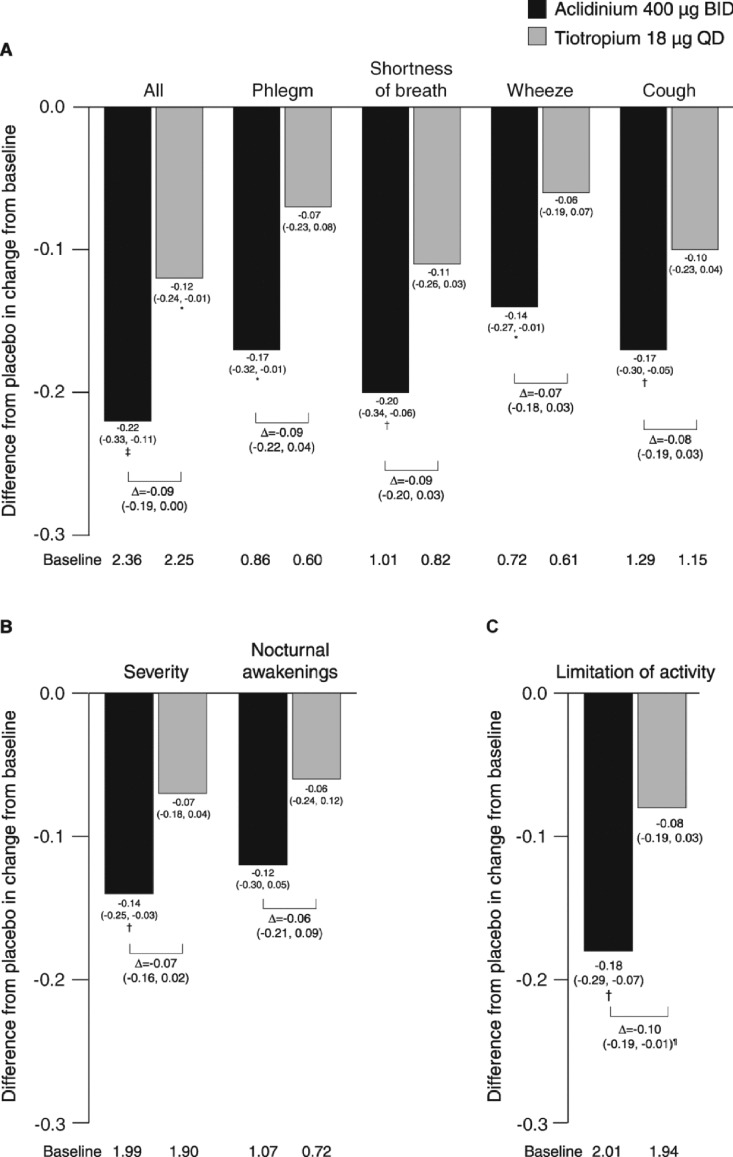
Figure 5.
Difference from placebo in change from baseline in (A) severity of early-morning symptoms, (B) severity of nighttime symptoms and number of nocturnal awakenings due to COPD symptoms, and (C) limitation of activity caused by COPD symptoms (COPD additional symptoms questionnaire) over 6 weeks (ITT population). Data reported as LS mean differences from placebo (ANCOVA). *p < 0.05; †p < 0.01; ‡p < 0.001 for aclidinium or tiotropium versus placebo. ¶p < 0.05 for aclidinium versus tiotropium. Severity of overall early-morning and nighttime symptoms rated on a 5-point scale from 1 = ‘did not experience any symptoms’ to 5 = ‘very severe’; individual morning symptoms rated on a 5-point scale from 0 = ‘no symptoms’ to 4 = ‘very severe’; limitation of activity rated on a 5-point scale from 1 = ‘not at all’ to 5 = ‘a very good deal’. ANCOVA, analysis of covariance; BID, twice daily; COPD, chronic obstructive pulmonary disease; ITT, intent-to-treat; LS, least squares; QD, once daily.