Figure 5.
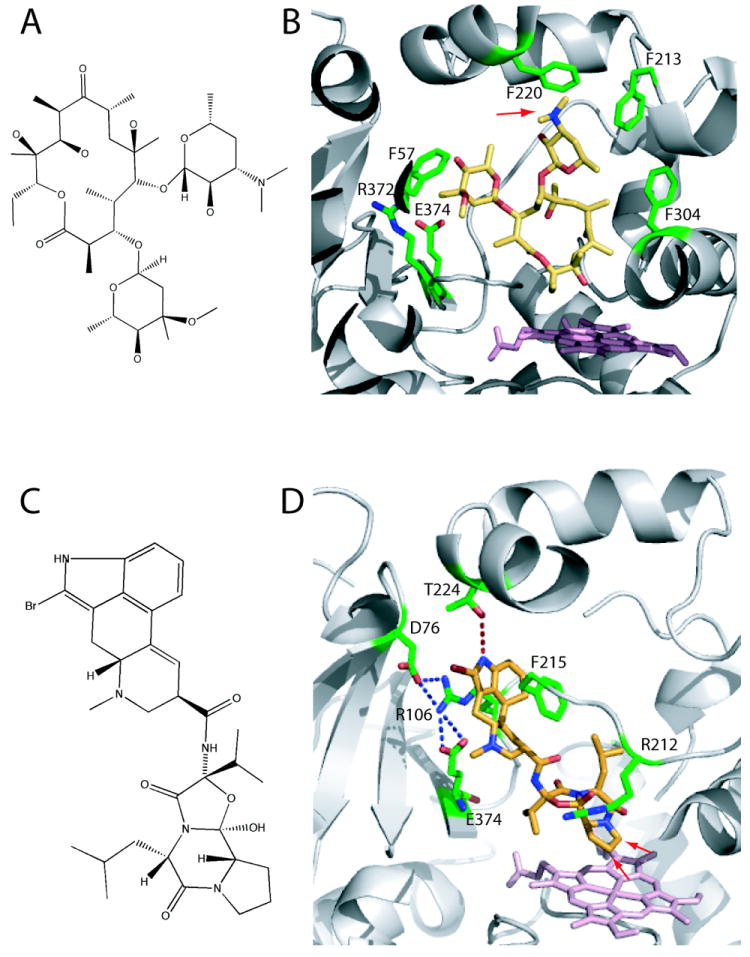
A, Chemical structure of erythromycin. B, Erythromycin bound to the active site of CYP3A4 (2JOD).38 The heme is in pink and residues within the van der Waals distance are in green. Erythromycin is bound in a non-productive mode because its demethylation site (indicated by an arrow) is 17 Å away from the heme. C, Chemical structure of BEC. D, BEC bound to the active site of CYP3A4 in a productive mode (3UA1 structure).31 The tripeptide moiety of BEC approaches the heme, with the two primary oxidations sites (indicated by arrows) being ~ 4 Å away from the heme iron. To allow BEC to access the heme, Arg212 adopts a different conformer. The lysergic group is sandwiched between the side chains of Arg106 and Phe215, and is hydrogen bonded to Thr224.
