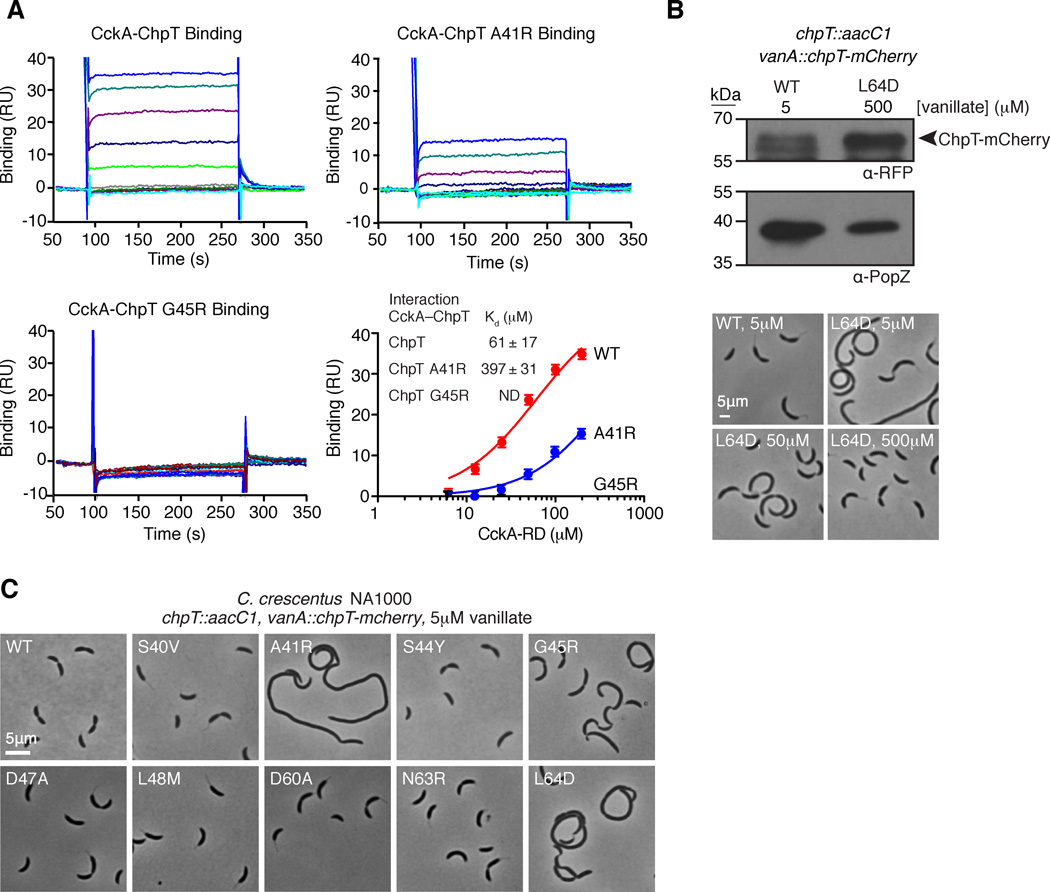
FIGURE 5.
A. Surface plasmon resonance demonstrates that His6-ChpT and His6-ChpT-A41R interact with CckA70–691, but His6-ChpT-G45R does not. Each SPR trace represents an increasing concentration of CckA-RD (1.6, 3.2, 6.3, 12.5, 25, 50, 100 and 200 µM). The mean response units (RU) from the CckA-ChpT interaction are plotted as a function of the CckA-RD concentration and fit to a one site specific binding model. Error bars represent the maximum deviation from the mean. B. ChpT point mutations along the putative RR binding interface affect Caulobacter crescentus morphology. Phase contrast micrographs of Caulobacter NA1000 strains whose sole chpT copy is either wild-type chpT-mcherry or a chpT-mcherry variant harboring a chpT RR binding mutation. C. Overexpression of chpT-mcherry-L64D overcomes cell morphology defects. Western blot analysis of ChpT-mCherry levels using an anti-RFP antibody confirms ChpT-mCherry L64D overexpression at 0.5mM vanillate relative to ChpT-mCherry expression levels at 5 µM vanillate. Probing the same samples with anti-PopZ sera provides a loading control. Phase contrast micrographs of chpT-mcherry-L64D cells reveal normal morphologies at 0.5 mM vanillate (see also Fig. S5).