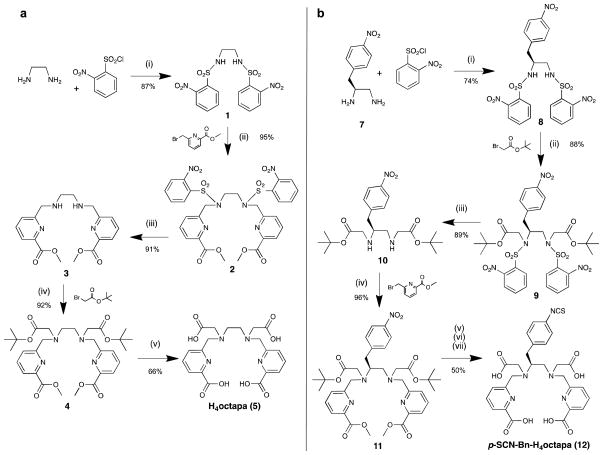
Scheme 1. Improved synthesis of chelators utilizing nosyl protection chemistry.
a, (i) THF, NaHCO3, 2-nitrobenzenesulfonyl chloride (2.2 equiv), RT, 24 h, 87%; (ii) DMF, Na2CO3, methyl-6-bromomethylpicolinate (2.2 equiv), 50 °C, 24 h, 95%; (iii) THF, thiophenol (2.2 equiv), K2CO3, RT, 72 h, 91%; (iv) MeCN, Na2CO3, tert-butylbromoacetate (2.2 equiv), 50 °C, 24 h, 92%; (v) 6 M HCl, reflux, 24 h, 66%. b, (i) THF, NaHCO3, 2-nitrobenzenesulfonyl chloride (2.2 equiv), 50 °C, 24 h, 74%; (ii) DMF, Na2CO3, tert-butylbromoacetate (2.2 equiv), 50 °C, 24 h, 88%; (iii) THF, thiophenol (2.2 equiv), K2CO3, RT, 72 h, 89%; (iv) MeCN, Na2CO3, methyl-6-bromomethylpicolinate (2.2 equiv), 50 °C, 24 h, 96%; (v) 5 mL of (1:1) Glacial acetic acid:3 M HCl, Pd/C (20 wt%), H2 (g) balloon, RT, 1 h; (vi) 6 M HCl, reflux, 24 h; (vii) thiophosgene in DCM (15 equiv), 3 M HCl, RT, 24 h, 50% over steps v-vii.