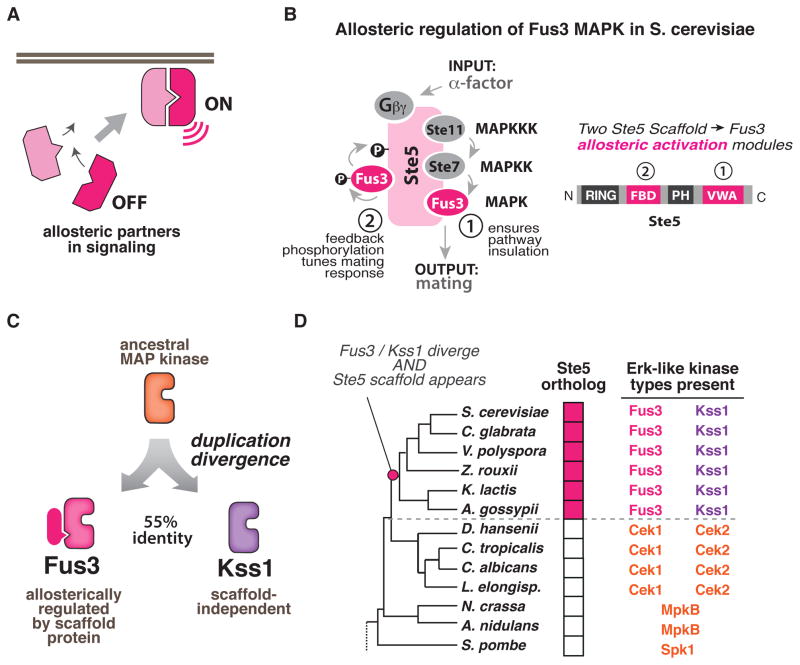
Figure 1. Fungal Erk kinase signaling repertoires provide a model system for biochemically interrogating the evolution of novel and divergent allosteric activation mechanisms.
(A) Allosteric interactions between signaling partners control when and where signaling molecules are activated in cells. (B) In the S. cer. mating MAP kinase pathway, two unique allosteric activities of the Ste5 scaffold regulate the MAP kinase Fus3 but not its paralog Kss1: (1) a VWA domain in Ste5 (Ste5-VWA) that is required to allosterically prime Fus3 for phosphorylation by the upstream MAPKK Ste7; and (2) a Fus3-binding domain (FBD) which stimulates Fus3 autophosphorylation as part of a negative feedback loop that shapes the morphological response of cells to mating pheromone. (C) Fus3 and Kss1 are Erk-like kinases that are 55% identical and arose from a duplication of an ancestral MAP kinase. (D) Abbreviated phylogeny of fungal species from Ascomycota with the signaling repertoire (number and types of ERK-like kinases present; presence or absence of Ste5 scaffold) indicated for each species (see also Fig. S1A–C).