Table 1.
Structure and growth-inhibitory activity of ITC and related compounds against B. cereus.
| # | Structure | MIC, μg ml−1
|
Synthetic protocol | |
|---|---|---|---|---|
| E. coli | B. cereus | |||
| Aliphatic ITC derivatives | ||||
| 8. | CH3-NCS | >128 | 128 | * |
| 22. | CH2=CH-CH2 –NCS | >128 | 128 | * |
| 7. | CH3CH2CH2CH2-NCS | >128 | 128 | * |
| Hydroxy- ITC derivatives | ||||
| 18. | HO -(CH2)4–NCS | >128 | 16 | * |
| 5. | HO -(CH2)5–NCS | >128 | 16 | * |
| 21. | HO -(CH2)6–NCS | >128 | 16 | * |
| 44. | HO -(CH2)7–NCS | >128 | 16 | * |
| 45. | HO -(CH2)8–NCS | >128 | 8 | I |
| Charged ITC derivative | ||||
| 6. | −2O3PO -(CH2)5 -NCS | 128 | >128 | III |
| Bifunctional ITC derivatives | ||||
| 38. | SCN -(CH2)4 –NCS | >64 | 2 | I |
| 39. | SCN -(CH2)5 –NCS | >64 | 2 | I |
| 40. | SCN -(CH2)6 –NCS | >64 | 2 | I |
| 41. | SCN -(CH2)7 -NCS | >64 | 2 | I |
| 42. | SCN -(CH2)8 -NCS | >64 | 2 | I |
| 43. | SCN-[(CH2)2 -O-]2 -(CH2)2 -NCS | >64 | 4 | I |
| 37. |
|
8 | 0.5 | I |
| 16. |
|
64 | 2 | I |
| Aromatic and heterocyclic ITC derivatives | ||||
| 4. |
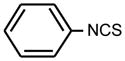
|
>128 | >128 | * |
| 1. |
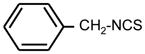
|
64 | 16 | * |
| 17. |
|
>128 | 16 | * |
| 3. |
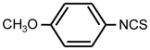
|
>128 | 64 | * |
| 2. |
|
64 | 8 | * |
| 20. |
|
128 | 8 | * |
| 27. |
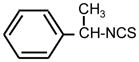
|
>128 | 16 | I |
| 19. |
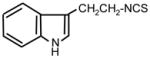
|
>128 | 8 | I |
| 34. |
|
>128 | 8 | II |
| 29. |
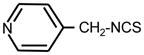
|
>128 | >128 | II |
| 30. |
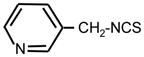
|
>128 | 8 | II |
| 31. |
|
>128 | 4 | II |
| 32. |
|
128 | 8 | II |
| 35. |
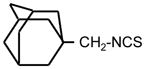
|
>128 | 8 | I |
| 14. |
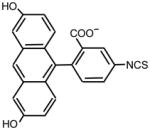
|
>128 | 64 | ** |
| 33. |
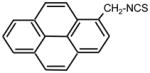
|
>128 | 0.5 | I |
| Control compounds | ||||
| 23. |
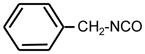
|
>128 | >128 | ** |
| 25. |
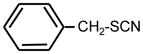
|
>128 | >128 | ** |
Known compounds
