Abstract
Background
For most patients with severely ankylosed hips, traditional surgical approaches do not provide sufficient exposure during THAs. We report our experience with a combined anterior and posterior approach using a lateral incision for total hip arthroplasty (THA) in patients with severe, spontaneous bony hip ankylosis.
Methods
Between January 2004 and December 2008, patients with severe, spontaneous bony hip ankylosis underwent THA via a combined anterior and posterior approach using a lateral incision.
Results
We included 47 patients (76 hips) with a mean age of 53 (range 22–72) years in our study. All surgeries were successful, and no significant postoperative complications occurred. The mean operative duration was 1.5 (range 1.3–1.7) hours, and mean blood loss was 490 (range 450–580) mL. The mean duration of follow-up was 5.5 (range 2–11) years. Harris hip score improved from 53 to 88 points postoperatively, and the outcome was good to excellent in 88.37% of cases. Heterotopic ossification occurred in 6 hips, and infection, which resolved with antibiotics, occurred in 1 patient.
Conclusion
This combined anterior and posterior approach to THA using a lateral incision in patients with severe, spontaneous ankylosis provides very good exposure, protects the abduction unit and results in good to excellent postoperative recovery.
Abstract
Contexte
Pour la plupart des patients atteints d’une grave ankylose de la hanche, les approches chirurgicales classiques n’exposent pas suffisamment l’articulation lors des interventions pour prothèse totale de la hanche (PTH). Nous faisons état de notre expérience d’une approche antéro-postérieure à l’aide d’une incision latérale pour la PTH chez des patients atteints d’une grave ankylose ostéoarticulaire spontanée de la hanche.
Méthodes
Entre janvier 2004 et décembre 2008, nous avons effectué des interventions pour PTH par l’approche antéro-postérieure à l’aide d’une incision latérale chez des patients présentant une grave ankylose ostéoarticulaire spontanée de la hanche.
Résultats
Nous avons inclus dans notre étude 47 patients (76 hanches) âgés en moyenne de 53 (entre 22 et 72) ans. Toutes les interventions ont réussi et on n’a eu à déplorer aucune complication postopératoire significative. La durée moyenne de l’intervention a été de 1,5 (entre 1,3 et 1,7) heure et la perte de sang moyenne a été de 490 (entre 450 et 580) mL. La durée moyenne du suivi a été de 5,5 (entre 2 et 11) ans. Le score de Harris pour la hanche s’est amélioré, passant de 53 à 88 points après l’intervention, et les résultats ont été de bons à excellents dans 88,37 % des cas. On a observé une ossification hétérotopique dans 6 hanches, et 1 patient a présenté une infection traitée avec succès par antibiothérapie.
Conclusion
Cette approche antéro-postérieure à l’aide d’une incision latérale pour la PTH chez des patients présentant une grave ankylose ostéoarticulaire spontanée offre une excellente visibilité. Elle protège le système d’abduction et permet un rétablissement postopératoire de bon à excellent.
Ankylosis, spontaneous fusion of a joint after injury or disease, is caused by a number of conditions, including rheumatoid arthritis, tuberculosis, septic arthritis, ankylosing spondylitis, severe osteoarthritis and hip trauma.1 Regardless of whether a patient has fibrous or bony ankylosis, the clinical manifestation is complete loss of both active and passive motion of the hip joint.2 Considering the negative impact on quality of life, an appropriate surgical intervention should be considered. Total hip arthroplasty (THA) has become the most common surgical procedure performed in developed nations.2,3 Good outcomes are reported following THA in patients with ankylosed hips;4 however, the procedure is technically demanding, and postoperative complications are not uncommon, particularly when compared with primary THAs.5
Common approaches to the nonankylosed hip include the anterior, posterior and lateral approaches. Each has its own advantages and disadvantages. For example, the Smith–Peterson approach cannot ideally expose the hip joint unless either the gluteus medius is partially detached from the greater trochanter or an additional trochanteric osteotomy is performed. The Watson–Jones approach offers poor operative exposure and requires partial destruction of the abductors, and the space posterior to the acetabulum is limited for intraoperative manipulation. The Hardinge approach provides limited exposure and thus is not suitable for patients with bony hip ankylosis, especially for those in whom there is difficulty exposing the posterior margin of the acetabulum. Finally, with the posterolateral approach, abduction of the hip is limited and anterior exposure of the acetabulum is relatively difficult.6
For most patients with severely ankylosed hips, traditional surgical approaches do not provide sufficient exposure during THAs.7–10 Femoral external rotation or adduction deformities are common with ankylosed hips, and thus a simple anterior or posterior approach cannot satisfactorily expose the surgical field. The purpose of this report is to relay our experience with a combined anterior and posterior approach using a lateral incision for THA in patients with severely ankylosed hips. We hypothesized that this combined approach would improve exposure of the hip joint capsule compared with both the posterior and anterior approaches and that it would result in good to excellent clinical outcomes.
Methods
Patient selection
Between January 2004 and December 2008, patients with spontaneous bony hip ankylosis undergoing THA were treated using a combined anterior and posterior approach by the same group of orthopedic surgeons at the Changhai Hospital, Shanghai, China. Surgical indications for THA included persistent low back pain, ipsilateral knee pain and instability, contralateral hip pain, nonfunctional hip fusion preventing ipsilateral knee arthroplasty, flexion deformity greater than 30°, adduction deformity greater than 10° and heterotopic fusion. Young patients requiring aesthetic or functional correction were also included. We excluded patients if there was either absence or severe fibrosis of the abductors owing to prior surgeries, recent signs of infection or marked atrophy of the quadriceps and/or abductors. We also excluded patients with fibrous ankylosis. Patients were consecutively enrolled, and they all provided informed consent. The ethics committee of the hospital approved our study protocol.
We calculated Harris hip scores for all patients both pre- and postoperatively (at the time of last follow-up). A score of 90–100 points was considered excellent, 80–89 points was considered good, 70–79 points was considered fair and a score of less than 70 points was considered poor.
Surgical procedure
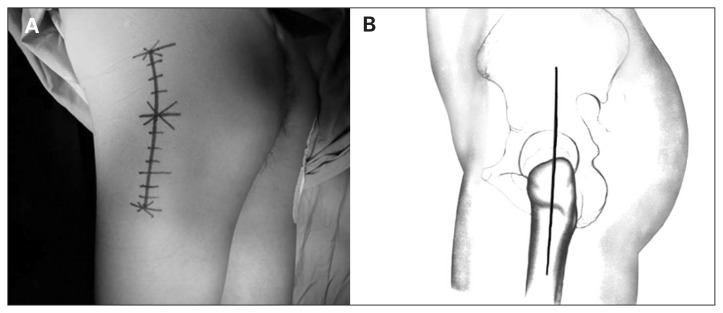
Patients were placed in the lateral recumbent position (Fig. 1A). The surgeon made a skin incision along the centre of the femoral shaft, with the superior end 8 cm above the tip of the greater trochanter and the inferior end 7 cm below the tip of the greater trochanter (Fig. 1B). Thus, the total incision length was 15 cm. The subcutaneous tissues were dissected to expose the fascia lata, which was then longitudinally split distally to proximally. The trochanteric bursa over the greater trochanter was incised, and then the gluteus maximus was cut proximally along the interval between the gluteus maximus and the tensor fascia lata to the proximal skin incision. The surgeon then made a 2 cm incision and bluntly dissected the fascia lata superiorly and inferiorly. Two thyroid retractors were used to slightly pull the tensor fascia lata to maintain tension. The posterior side was exposed first (Fig. 2A). The operating table was rotated toward the prone position (away from the surgeon) by 15°, and gauze was packed inside the deep surface of the gluteus maximus to control bleeding. The gluteus maximus and fascia lata were retracted posteriorly to expose the gluteus medius and external rotators. In patients with ankylosed hips, it is not possible to flex the knee joint to 90° and internally rotate the hip joint to make the external rotators tense. Therefore, the piriformis tendon was carefully elevated with a long, curved vascular clamp at the junction of the posterior margin of the insertion site of the gluteus medius and the greater trochanter. The piriformis was then incised at the insertion site of the tendon. Along the intertrochanteric fossa and the posterior side of the greater trochanter, the superior gemelluse, obturator internus, inferior gemellus and the insertion site of the quadratus femoris were cut close to the bone with an electric scalpel. The surgeon further dissected the external rotators on the posterior and inferior surface of the joint capsule with a long-handled periosteal elevator. The retractor was adjusted to expose the posterior and inferior joint capsule. The junction between the deep part of the gluteus minimus and the superficial surface of the upper joint capsule was bluntly dissected with a long-handled periosteal elevator, and the gluteus minimus and the gluteus medius were retracted superiorly with a Hoffman retractor. Most parts of the superior, posterior and inferior joint capsule were removed, and the attached soft tissues were removed with an electric scalpel along the femoral neck. Large gauze was used to pack the incision site to complete the posterior exposure.
Fig. 1.
(A) The patients were placed in a lateral recumbent position with the diseased limb upward. The pubic symphysis and sacrum were fixed anteriorly and posteriorly, and the motion of the lower limb was preserved. (B) Illustration of the surgical incision with reference to the greater trochanter.
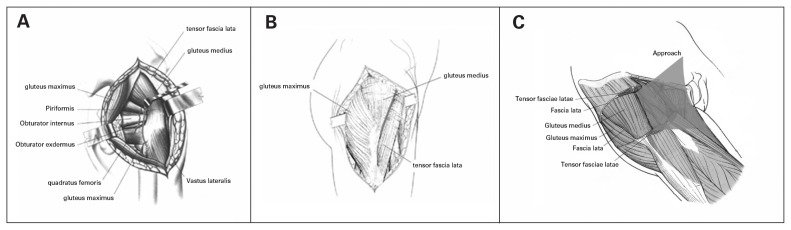
Fig. 2.
(A) The operating table was rotated away from the surgeon by 15°, and the posterior joint capsule of the hip joint was exposed. (B) The operating table was then rotated toward the surgeon by 15° to allow access to the anterior structures of the hip joint. (C) The anterior hip joint capsule was exposed by approaching the spatium intermusculare between the gluteus medius and tensor fascia lata.
To expose the anterior joint capsule, the operating table was rotated toward the supine position (toward the surgeon) by 15°, and the fascia lata was retracted anteriorly and posteriorly. An incision was made along the junction between the tensor fascia lata and the gluteus medius (Fig. 2B). The gluteus medius was retracted posteriorly, the fascia lata was pulled anteriorly, and the anterior part of the joint capsule was exposed after releasing the soft tissue adhering to the femoral side of the joint capsule. Dissection was performed along the space between the gluteus medius and tensor fascia lata toward the joint capsule (Fig. 2C). The soft tissue on the anterior joint capsule was then dissected with a periosteal elevator, and the anterior and inferior aspect of the joint capsule was exposed completely by adjusting the position of the retractors. A Hoffman retractor was inserted between the deep part of the gluteus minimus and the superficial surface of the upper joint capsule to pull the gluteus minimus and the gluteus medius superiorly. Injury to the superior gluteal nerve due to excessive retraction was avoided. The anterior joint capsule and remnants of the superior and inferior joint capsule were removed. To improve exposure, the femoral vastus lateralis was split in the direction of the femur. The ankylosed hip joint was totally exposed and the external abductors (i.e., gluteus medius and minimus) were preserved.
The femoral neck osteotomy (Figs. 3 and 4) was achieved in 2 steps. First, via the anterior approach, the osteotomy was performed 2 cm posteroinferior to the junction of the femoral head and neck with an oscillating saw. Via the posterior approach, the osteotomy was performed 2 cm interoinferior to the junction of the femoral head and neck. A V shape was formed by the anterior and posterior clefts to make the previously ankylosed hip a mobile hip. Then, a standard femoral neck osteotomy was performed 1 cm superior to the lesser trochanter. The sciatic nerve (identified via the loosely packed adipose tissue surrounding the nerve) was located in the gap between the gluteus maximus and the group of external rotation muscles. Under normal circumstances, no routine release was performed; however, for patients with severely contracted joint capsules, the soft tissue surrounding the sciatic nerve was released by 4–5 cm along the nerve to provide additional room. The newly formed hip joint was moved repeatedly and the tense soft tissue, particularly the inferior and medial soft tissue, around the hip joint was released.
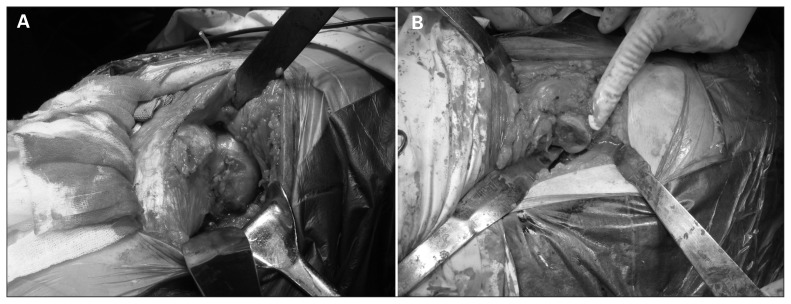
Fig. 3.
Result of the femoral neck exposure. The abductors were preserved after exposing the femoral neck by gently applying retractors after the combined anterior and posterior approach. There was no need to perform an osteotomy of the greater trochanter in any of the included patients.
Fig. 4.
(A) The femoral neck was exposed via a combined anterior and posterior approach. (B) The femoral head has been amputated.
The exact location of the acetabulum was identified with the assistance of preoperative radiographs and remaining stumps following the femoral neck osteotomy. This is an important step for ankylosed hips with in situ fusion; however, it does not work for the ankylosed hip with deformed fusions. A small curved osteotome was then inserted approximately 2 cm posterior and inferior to the junction of the femoral head and neck in the anterior surgical field. Preoperative radiographs faciliated proper positioning. The knee joint was flexed 90°, and the hip joint was internally rotated to expose the acetabulum. The acetabulum was reamed at 45° inclination and 15° anteversion, and an acetabular trial insert was placed. Next, the femoral canal was reamed and a suitable femoral neck prosthesis was selected. The femoral components used were coated anatomic medullary locking (DePuy), and the acetabular components used were Duraloc (Depuy). Of the implants, 9 were cemented and 67 were noncemented. Hip joint range of motion and soft tissue tension were observed after reduction. If the soft tissue, especially the gluteus medius, was tense, then a secondary release was made along the superior margin of the acetabulum until the result was satisfactory. Hip joint reduction was performed, and again the hip joint range of motion and soft tissue tension were assessed. The surgical wound was closed via a layer-by-layer method, and continuous drainage with negative pressure was applied.
We defined blood loss as the volume of intraoperative suction plus the change in weight of the sponges used (postoperative sponge weight – preoperative sponge weight), with the sum divided by the density of blood (1.055 g/cm3). Postoperative blood loss through drainage was not included.
Postoperative management and follow-up
Preoperative parenteral antibiotics and prophylaxis for deep vein thrombosis (4 mg/d of fraxiparine, subcutaneous) were administered to all patients. Postoperatively, the affected limb was placed in a neutral abduction position and a special shoe was applied to avoid adduction and internal rotation, as this can lead to the development of early postoperative hip joint dislocation. A pillow was placed under the knee to flex the knee joint 30°, and a triangular pillow was placed between the legs. The drain was removed 24–48 hours postoperatively.
Non–weight bearing exercise and crutch ambulation were initiated 1 and 2 weeks postoperatively, respectively. Partial weight-bearing began 6–8 weeks postoperatively; it was delayed to provide sufficient time to ensure maximal soft-tissue healing because the extent of the soft-tissue dissection in ankylosed hips is greater than in healthy hips. We obtained radiographs before discharging the patients.
All patients underwent follow-up examination 1, 3 and 6 months after discharge and yearly thereafter. Two independent observers, 1 surgeon and 1 physician not involved in the arthroplasty, performed the follow-up examinations. Pain, function, deformities and range of motion using the Harris hip score were assessed at the last follow-up, and the sum range of motion was measured based on flexion, extension, abduction, internal adduction internal and external rotation movements.
Results
We included 47 patients (76 hips) in our study: 29 (48 hips) were men, and the mean patient age was 53 (range 22–72) years. Patient characteristics are summarized in Table 1. All cases were primary THAs in patients with severe, spontaneous ankylosed hip joints. A representative case is shown in Figure 5. The mean operative duration was 1.5 (range 1.3–1.7) hours, and mean blood loss was 490 (range 450–580) mL. Fractures limited to the proximal femoral metaphysis occurred in 5 patients, and wiring was required in 2 of them. Immediate postoperative radiographs revealed excellent location of all prostheses, as the rotation centre of the hip joints were reconstructed and the fixation status of the prostheses was deemed acceptable.
Table 1.
Demographic and clinical characteristics of patients undergoing total hip arthroplasty for ankylosed hip joints
| Characteristic | No. (%)* |
|---|---|
| No. of patients (no. of hips) | 47 (76) |
| Sex | |
| Male | 29 (62) |
| Female | 18 (38) |
| Affected side | |
| Right | 36 (77) |
| Left | 40 (85) |
| Age, mean (range) yr | 53 (22–72) |
| Duration of disease at time of surgery, mean (range) yr | 7.5 (5–9) |
| Patient follow-up time, mean (range) yr | 5.5 (2–11) |
| Presence of hip flexion contracture | 67 (88) |
| Cause of ankylosis | |
| Rheumatoid arthritis | 9 (19.1) |
| Tuberculosis of the hip | 6 (12.9) |
| Sequela of the septic arthritis | 7 (14.9) |
| Anklylosing spondylitis | 16 (34.0) |
| Osteoarthritis | 5 (10.6) |
| Hip trauma | 4 (8.5) |
Unless otherwise indicated.
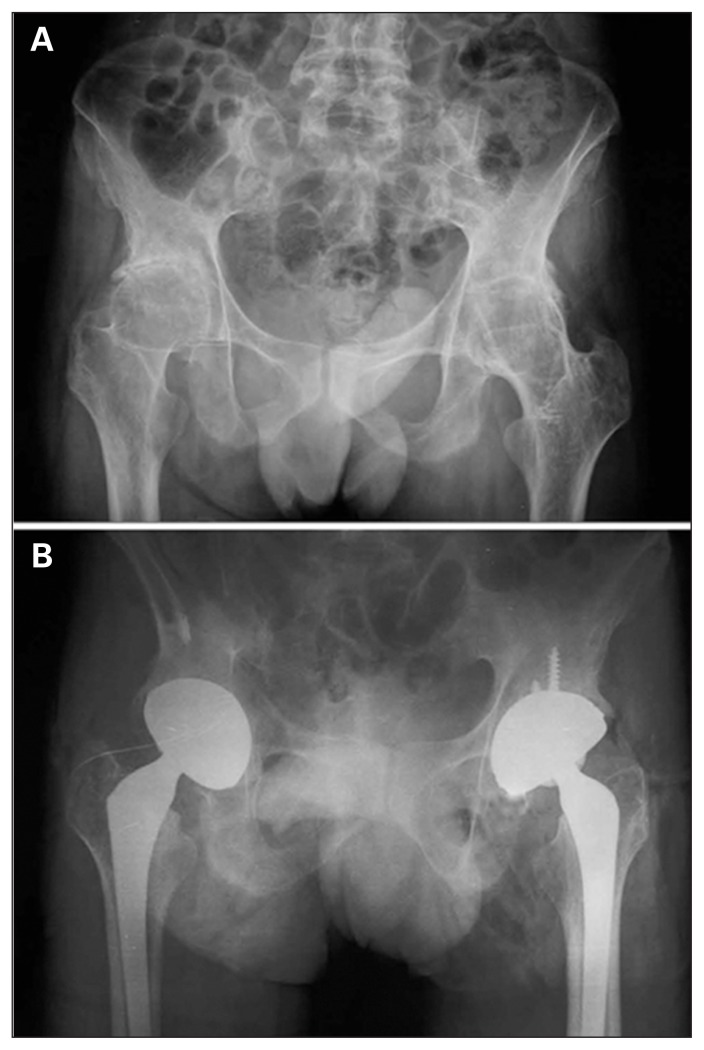
Fig. 5.
(A) Preoperative radiographs of a 35-year-old man. He had ankylosing spondylitis associated with bilateral ankylosed hip joints. Because the patient had lower extremity deformities, the pelvis could not be oriented in an ideal anteroposterior position for radiographs. The range of motion in every direction was 0 in both hip joints and the patient experienced severe pain in both hip joints. (B) Anteroposterior radiographs of the same patient 3 years postoperatively. The position of the prosthesis was excellent. The pain in both hip joints was completely resolved, and 89° and 100° flexion was achieved in the left and right hip joints, respectively. The patient’s gait was normal postoperatively.
The mean duration of follow-up was 5.5 (range 2–11) years. Four patients (6 hips) were lost to follow-up after discharge. In the remaining 43 patients, there were no postoperative dislocations or hip impingements. The mean Harris hip score increased from 53 points preoperatively to 88 points postoperatively. The outcome was excellent in 14 patients, good in 24, fair in 4 and poor (because of infection) in 1 patient. In total, 88.37% of the outcomes were considered good to excellent.
Postsurgically, 4 hips were associated with mild pain and discomfort, which was successfully managed via patient-controlled analgesia. In 1 case there was pain in the middle and upper thigh, which gradually improved over 3–4 weeks. Heterotopic ossification occurred in 6 hips: 3 were grade I and the rest were grade II according to the Brooker classification. Hip function was not affected in any of these patients. Mild nerve traction injury occurred in 2 patients; it presented as numbness of the skin over the lateral aspect of the limb and foot on the operated side. Nonsteroidal anti-inflammatory drugs and vitamin B were prescribed, and the condition resolved in both patients within 6 months of surgery. Neither patient had evidence of motor neuron injury.
Postoperative measurements of the leg revealed a leg length difference of ≤ 1 cm in all but 1 patient, in whom 1 limb was lengthened by 1.3 cm and the other limb shortened by 0.9 cm. The patient had difficulty walking, which was resolved by adjusting the patient’s shoes. The main type of nerve injury was sciatic nerve palsy, and all patients were negative for Trendelenburg gait during the last follow-up visit. Preoperatively, flexion contracture was noted in 67 (88%) hips, the mean deformity angle was 37° (range 12°–89°), and range of motion was 0°. At the last follow-up, mean range of motion was 119.7° (range, 100°–135°) in the replaced joints (Table 2).
Table 2.
Mean patient hip movements at various time points postoperatively
| Function | Postoperative time; mean ± SD | ||
|---|---|---|---|
| 8 wk | 12 wk | 6 mo | |
| Flexion, ° | |||
| Left | 65.08 ± 8.16 | 90.57 ± 5.55 | 115.54 ± 9.74 |
| Right | 68.13 ± 7.09 | 90.66 ± 6.82 | 111.66 ± 12.00 |
| Extension, ° | |||
| Left | 7.97 ± 1.91 | 10.51 ± 2.45 | 19.84 ± 6.04 |
| Right | 8.41 ± 2.01 | 10.59 ± 1.92 | 20.63 ± 7.49 |
| Abduction, ° | |||
| Left | 24.38 ± 4.52 | 27.92 ± 2.69 | 34.73 ± 4.24 |
| Right | 24.09 ± 4.11 | 28.09 ± 2.47 | 34.53 ± 2.65 |
SD = standard deviation.
Discussion
This report describes the results of a THA technique in 47 patients with severe, spontaneous ankylosed hip joints using a combined anterior and posterior approach via a lateral incision. This combined approach preserves the abductors with no damage to either the gluteus medius or gluteus minimus, thereby improving postoperative joint function; does not increase the length of the surgical incision, but rather alters the position of the surgical field for performing both the anterior and posterior procedures; clearly exposes the acetabulum and adjacent tissues; and allows the incision to be extended superiorly or inferiorly at any time during the surgery, if indicated.
This approach is markedly different from the standard surgical approaches to THA. Two common approaches for THAs are a posterolateral approach (e.g., the Gibson or Osborne approaches) and a lateral approach (e.g. the Ollier approach).2,5 When performing a primary THA, these approaches are simple and convenient, the anatomic structures are distinct and surgical exposure is sufficient to achieve a satisfactory outcome. In ankylosed hip joints, however, these approaches are not optimal. The Gibson approach easily exposes the femoral side, but the external rotators and posterior joint capsule are difficult to expose if the ankylosis is associated with an external rotation deformity of the femur. Furthermore, the exposure and release of the anterior joint capsule is difficult if an adduction deformity exists. The Watson–Jones approach does not clearly expose the medial and inferior margin of the acetabulum. Thus, extensive soft tissue release (removing most parts of the insertion site of the gluteus medius) or a greater trochanter osteotomy are often inevitable, which can contribute to the development of postoperative complications, such as muscle weakness in the abductors, superior gluteal nerve injury (innervating the tensor fascia lata, gluteus medius and minimus) and greater trochanter nonunion.11 The Hardinge approach requires no greater trochanter osteotomy, and the approach maintains the integrity of the gluteus medius. However, the approach provides limited exposure, and thus is not suitable for patients with bony hip ankylosis, especially for those in whom there is difficulty exposing the posterior margin of the acetabulum, which would affect placement of the acetabular prosthesis.
A transtrochanteric approach is the most commonly used approach for ankylosed hips.12,13 However, it can result in massive bleeding and has a relatively long operative duration. Furthermore, fixation of the greater trochanter is difficult, heterotopic ossification is severe, postoperative local pain occurs, bursitis of the greater trochanter can develop, and the rate of trochanteric nonunion following the transtrochanteric approach has been reported to be 0.8%–5%.14,15
The combined approach presented herein may appear similar to the technique described for periacetabular osteotomies and to some of the surgeries retrospectively reviewed by Bhan and colleagues,16 and to the “minimally invasive 2-incision approach” described by Berger;17 however, there are some major differences. The Berger method approaches the hip between the tensor fascia lata and the sartorius muscle, and the rectus femoris is pulled inwardly to expose the capsula articularis coxae. This method does not adequately expose the acetabulum in patients with severely ankylosed hips. In addition, Bhan and colleagues and Berger reported the use of fluoroscopy and 2 skin incisions for each hip.
As our study was not a controlled clinical trial, directly comparing these results with a similar group of patients treated via alternate approaches is difficult. As demonstrated in Table 3, the published studies on THA in patients with ankylosed hips include variable etiologies of the ankylosis and variable outcome measures, use different types of prostheses and may or may not involve performing a trochanteric osteotomy.5,16,18–23 Nonetheless, some comparisons can be made, as Harris hip scores have been reported in several studies. In our study, the Harris hip score improved by a mean of 35 points. This is marginally greater than the improvement reported by Bhan and colleagues ( 33.1),16 Kim and colleagues (26.9)18 and Abdel-Aal and colleagues (34),19 and markedly greater than the improvement reported by Rajaratnam and colleagues (13).22 However, the improvement in our study is notably lower than that reported by Bangjian and colleagues (71).23 In addition, postoperative complications in our study were minimal compared with those noted in the other published studies. For example, nerve injury to the sciatic, femoral and common peroneal nerves were reported in a number of studies, as were deep vein thrombosis, aseptic loosening and deep infections.
Table 3.
Summary of key studies of total hip arthroplasty in patients with ankylosed hips
| Study | Patients | Surgical approach | Outcomes |
|---|---|---|---|
| Present study | 47 patients (76 hips) with spontaneous ankylosis. | A combined anterolateral and posterolateral approach via a lateral incision. Mean follow-up was 5.5 years. | Harris hip score improved from 53 to 88 points postoperatively. The outcome was excellent to good in 88.37% of cases. Femoral prosthesis subsidence occurred in 3 hips. The maximum subsidence was 2 mm. No treatment was required as there were no symptoms related to the subsidence. Heterotopic ossification occurred in 6 hips. |
| Bhan et al.16 | Retrospective review of 54 patients (92 hips) who underwent cementless THA for bony ankylosis due to ankylosing spondylitis. | Posterior approach. Mean follow-up was 8.5 years. | Harris hip scores improved from 49.5 to 82.6. Complications included pain (n = 10), anterior dislocation (n = 4), sciatic nerve palsy (n = 1), revision arthroplasty due to aseptic loosening (n = 13). |
| Kim et al.18 | 12 patients with bilateral anyklosis (24 hips). | All surgeries performed by a single surgeon via the standard transtrochanteric approach. | Harris hip scores increased from 55.4 to 82.3. Complications included osteolysis (n = 3) and loosening (n = 2), and 11 cups were outside the safe ranges of Lewinnek. |
| Abdel-Aal et al.19 | 12 patients (15 hips) with surgically (n = 5) and spontaneously fused hips (n = 7). | Hardinge approach. | Harris hip scores in all patients improved from 42 preoperatively to 76 postoperatively. Complications included femoral artery injury due to Hofmann retractors in front of the acetabulum, 1 failed THA. |
| Joshi et al.5 | 103 patients underwent 181 THAs for ankylosing spondylitis. | Either direct lateral approach via trochanteric osteotomy or the Hardinge approach. All patients received cemented low-friction THAs. | No Harris hip scores were reported. Instead, authors indicated that 96% of hips had an excellent (low) pain score and 29.25 of the hips had normal or near-normal function. |
| Rutz et al.20 | 22 patients (22 ankylosed hips) secondary to posttraumatic osteoarthritis, coxitis, hip dysplasia and primary osteoarthritis. | 19 conversions were via a lateral approach and 3 were via an anterolateral approach; 20 patients were available at a mean follow-up of 13.2 years. | Harris hips scores postoperatively were 84.9 (presurgical scores were not cited). Complications occurred in 30% of patients and included transient apraxia of the femoral nerve, a lesion of the sciatic nerve, 2 deep infections, and aseptic loosening of 2 femoral stems. |
| Hamadouche et al.21 | 45 patients (45 hips) underwent THA for treatment of spontaneous ankylosis (n = 20) and postoperative ankylosis (n = 35). Cases of ankylosing spondylitis and fibrous ankylosis were excluded. | Lateral approach with a standard trochanteric osteotomy. | Merle d’Aubigné hip scores increased from 11.3 points preoperatively to 16.5 points at the time of last follow-up. Satisfactory hip function was achieved in 41 (91%) patients. Complications included deep vein thrombosis (n = 2), common peroneal nerve palsy (n = 1), and periprosthetic and inguinal abscess 1 year postoperatively (n = 1). |
| Rajaratnam et al.22 | 15 patients (16 hips) undergoing cementless THA to treat spontaneous ankylosis (including ankylosing spondylitis) or surgical arthrodesis. | Hardinge approach in 5 patients and the remaining 10 patients had a posterior approach with an enhanced posterior repair. Median follow-up was 10.75 years. | Harris hip scores improved from 70 to 83 and Merle d’Aubigné hip scores had a score of 4.8 for pain, 3.9 for mobility, and 4.4 for function. The overall Merle d’Aubigné hip score postoperatively was a mean of 13. Aseptic loosing requiring acetabular cup revision occurred in one patient, and deep vein thrombosis occurred in another. |
| Bangjian et al.23 | 12 patients with bilateral ankylosed hip joints (24 hips) caused by late ankylosing spondylitis. | The most appropriate approach depending on patient anatomy and the incision was via the posterolateral approach. Mean follow-up was 4.2 years. | All hip joint function improved, and flexion deformity was corrected. Flexion ranges were 75–105° (mean 84.4°) and extension ranges were 10° to 20° (mean, 18.7°). Harris hip scores ranged from 15.21 points preoperatively to 86.25 points postoperatively. No patient experienced hip pain postoperatively, and preoperative knee and lower back pain were clearly relieved postoperatively. |
THA = total hip arthroplasty.
The mean range of motion (119.7°) in our study is better than that reported in many primary THA studies. This may be because of differences in the procedures and the subjectivity in measurements. In our practice, range of motion is in part determined by evaluating what kinds of daily activities the patients can perform. For patients who could squat for toileting (and in this study all patients could do so), performing such action would require at least 120° of hip flexion. After a primary THA, patients would likely be able to satisfy the criterion of sitting on stools, which typically requires 100°–120° range of motion.
Despite being unable to directly compare the published studies on THA in ankylosed hips, our study presents superior outcomes in terms of low dislocation rate, good hip abduction function and full correction of preoperative hip deformities than the other series listed in Table 3. Futhermore, the approach described herein optimizes the prosthetic insertion by fully exposing the acetabular and femoral sides of surgical fields and prevents excessive soft tissue traction that could lead to blunt soft tissue injury. Finally, this approach avoids injuring the gluteal musculature that are essential in postoperative recovery of gait and hip abduction.
To ensure successful outcomes using this approach, a number of key points should be considered. First, be particularly diligent about protecting the gluteus medius and minimus and avoiding excessive traction of the gluteus medius and minimus to protect the superior gluteal nerve. Next, during the femoral neck osteotomy, protect the peripheral soft tissue. Third, repair the anterior and posterior soft tissues around the hip joint carefully, as surgical release leads to the loss of the stabilizing tissues, such as the joint capsule and tendons. Finally, when preparing the acetabulum and femur, protect the peripheral muscles by packing with gauze to avoid seeding bone marrow components into the peripheral muscles. This may decrease the incidence of heterotopic ossification.
Limitations
As noted, a limitation of this study is that it lacked a control group. In addition, the sample was relatively small and the follow-up relatively short. Finally, the Harris hip score, although commonly used, has many limitations as a research tool. However, any scoring method has limitations, and the Harris hip score has been adopted by our institution as a standard for assessment. Therefore, we used this method for the purpose of standardization and uniformity in our collected data.
Conclusion
This combined anterior and posterior approach using a lateral incision provides very good exposure of ankylosed hip joints, protects the abduction unit and results in good to excellent postoperative recovery. This THA approach should be considered in the management of patients with 1 or more naturally ankylosed hips.
Footnotes
Competing interests: None declared.
Contributors: Zhiwei Wang designed the study. J. Li, Zhiwei Wang, Y. Wu and W. Xu acquired the data, which Zhiwei Wang, M. Li, Y. Wu and Zimin Wang analyzed. J. Li and Zimin Wang wrote the article, which all authors reviewed and approved for publication.
References
- 1.Kim YH, Oh SH, Kim JS, et al. Total hip arthroplasty for the treatment of osseous ankylosed hips. Clin Orthop Relat Res. 2003;414:136–48. doi: 10.1097/01.blo.0000081935.75404.7f. [DOI] [PubMed] [Google Scholar]
- 2.Sathappan SS, Strauss EJ, Ginat D, et al. Surgical challenges in complex primary total hip arthroplasty. Am J Orthop. 2007;36:534–41. [PubMed] [Google Scholar]
- 3.Mizra SB, Dunlop DG, Panesar SS, et al. Basic science considerations in primary total hip replacement arthroplasty. Open Orthop J. 2010;4:169–80. doi: 10.2174/1874325001004010169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kilgus MD, Amstutz HC, Wolgin MA, et al. Joint replacement for ankylosed hips. J Bone Joint Surg Am. 1990;72:45–54. [PubMed] [Google Scholar]
- 5.Joshi AB, Markovic L, Hardinge K, et al. Total hip arthroplasty in ankylosing spondylitis: an analysis of 181 hips. J Arthroplasty. 2002;17:427–33. doi: 10.1054/arth.2002.32170. [DOI] [PubMed] [Google Scholar]
- 6.Palan J, Beard DJ, Murray DW, et al. Which approach for total hip arthroplasty: Anterolateral or posterior? Clin Orthop Relat Res. 2009;467:473–7. doi: 10.1007/s11999-008-0560-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bottner F, Delgado S, Sculco TP. Minimally invasive total hip replacement: the posterolateral approach. Am J Orthop. 2006;35:218–24. [PubMed] [Google Scholar]
- 8.Bertin KC, Röttinger H. Anterolateral mini-incision hip replacement surgery. A modified Watson-Jones approach. Clin Orthop Relat Res. 2004;429:248–55. [PubMed] [Google Scholar]
- 9.Basad E, Ishaque B, Stürz H, et al. The anterolateral minimally invasive approach for total hip arthroplasty: technique, pitfalls, and way out. Orthop Clin North Am. 2009;40:473–8. doi: 10.1016/j.ocl.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Ritter MA, Harty LD, Keating ME, et al. A clinical comparison of the anterolateral and posterolateral approaches to the hip. Clin Orthop Relat Res. 2001;385:95–9. doi: 10.1097/00003086-200104000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Amstutz HC, Dennis N. Total joint replacement for ankylosed hips. Indications, technique, and preliminary results. J Bone Joint Surg Am. 1975;57:619–25. [PubMed] [Google Scholar]
- 12.Kilgus DJ, Amstutz HC, Wolgin MA, et al. Joint replacement for ankylosed hips. J Bone Joint Surg Am. 1990;72:45–54. [PubMed] [Google Scholar]
- 13.Kevin B, Cleveland KB. Campbell’s Operative Orthopaedics. 11th ed. 1 pt VII. Philadelphia (PA): Mosby Elsevier; 2007. Nontraumatic soft tissue disorders. [Google Scholar]
- 14.Schinsky MF, Nercessian OA, Arons RR, et al. Comparison of complications after transtrochanteric and posterolateral approaches for primary total hip arthroplasty. J Arthroplasty. 2003;18:430–4. doi: 10.1016/s0883-5403(03)00144-x. [DOI] [PubMed] [Google Scholar]
- 15.Cashman JP, Cashman WF. Comparison of complications in transtrochanteric and anterolateral approaches in primary total hip arthroplasty. Orthopedics. 2008;31:1085. doi: 10.3928/01477447-20081101-04. [DOI] [PubMed] [Google Scholar]
- 16.Bhan S, Eachempati KK, Malhotra R. Primary cementless total hip arthroplasty for bony ankylosis with ankylosing spondylitis. J Arthroplasty. 2008;23:859–66. doi: 10.1016/j.arth.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Berger RA. Total hip arthroplasty using the minimally invasive two-incision approach. Clin Orthop Relat Res. 2003;(417):232–41. doi: 10.1097/01.blo.0000096828.67494.95. [DOI] [PubMed] [Google Scholar]
- 18.Kim YL, Shin SI, Nam KW, et al. Total hip arthroplasty for bilaterally ankylosed hips. J Arthroplasty. 2007;22:1037–1041. doi: 10.1016/j.arth.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Aal A, Bakr H, Mahran M. Total hip arthroplasty for fused hips. Orthopedics. 2010;33:400–4. doi: 10.3928/01477447-20100429-19. [DOI] [PubMed] [Google Scholar]
- 20.Rutz E, Schäfer D, Valderrabano V. Total hip arthroplasty after hip joint ankylosis. J Orthop Sci. 2009;14:727–31. doi: 10.1007/s00776-009-1390-3. [DOI] [PubMed] [Google Scholar]
- 21.Hamadouche M, Kerboull L, Meunier A, et al. Total hip arthroplasty for the treatment of ankylosed hips: a five to twenty-one-year follow-up study. J Bone Joint Surg Am. 2001;83-A:992–8. doi: 10.2106/00004623-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Rajaratnam SS, Sexton SA, Waters TS, et al. Long term results of cementless total hip replacement for reversal of hip ankylosis. Hip Int. 2009;19:120–7. doi: 10.1177/112070000901900207. [DOI] [PubMed] [Google Scholar]
- 23.Bangjian H, Peijian T, Ju L. Bilateral synchronous total hip arthroplasty for ankylosed hips. Int Orthop. 2011 Jul 13; doi: 10.1007/s00264-011-1313-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]