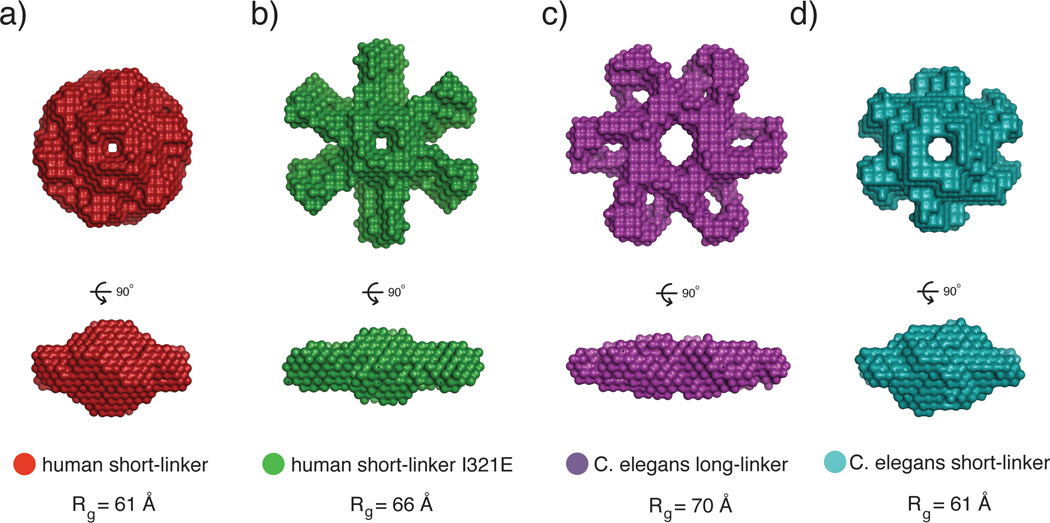
Figure 4.
SAXS analysis of the CaMKII holoenzyme. a) The human short-linker construct, used for the crystallization of the full-length holoenzyme, has a compact SAXS envelope. b) A mutation (I321E) introduced to the short-linker construct at the docking site between the kinase and hub domain shows a significantly larger SAXS envelope. This may represent an extended form of the autoinhibited holoenzyme where the kinases are popped out from the hub domain. Additionally, the I321E has a left-shifted EC50 value for CaM binding, which corroborates the model that the extended conformation is more accessible to CaM than the compact conformation observed in the crystal structure. c,d) C. elegans CaMKII also converts from a large to small SAXS envelope when the variable linker is shortened. These may also represent the extended and compact conformations of CaMKII, respectively. This figure was adapted from (35).