Abstract
Background & objectives:
All colonizing and invasive staphylococcal isolates may not produce biofilm but may turn biofilm producers in certain situations due to change in environmental factors. This study was done to test the hypothesis that non biofilm producing clinical staphylococci isolates turn biofilm producers in presence of sodium chloride (isotonic) and high concentration of glucose, irrespective of presence or absence of ica operon.
Methods:
Clinical isolates of 100 invasive, 50 colonizing and 50 commensal staphylococci were tested for biofilm production by microtiter plate method in different culture media (trypticase soy broth alone or supplemented with 0.9% NaCl/ 5 or 10% glucose). All isolates were tested for the presence of ica ADBC genes by PCR.
Results:
Biofilm production significantly increased in the presence of glucose and saline, most, when both glucose and saline were used together. All the ica positive staphylococcal isolates and some ica negative isolates turned biofilm producer in at least one of the tested culture conditions. Those remained biofilm negative in different culture conditions were all ica negative.
Interpretation & conclusions:
The present results showed that the use of glucose or NaCl or combination of both enhanced biofilm producing capacity of staphylococcal isolates irrespective of presence or absence of ica operon.
Keywords: Biofilm production, glucose, ica operon, NaCl, staphylococci
Staphylococci are common cause of hospital acquired infections and biofilm is one of its important virulence factors1,2. Its production is dependent on polysaccharide intracellular adhesin (PIA) synthesis. Regulation of PIA synthesis and biofilm production by microbes is a complicated process and is influenced by many factors, e.g. constitutional microbial factors, environmental factors and in clinical situations host proteins, etc. The enzymes involved in PIA synthesis are encoded by the ica operon comprising icaA, icaD, icaB, and icaC genes. Expression of the ica operon and formation of biofilm are highly variable3 and ica negative biofilm positive strains of staphylococci are also known to have alternative regulatory mechanism of biofilm formation4. All colonizing and invasive staphylococcal isolates may not produce biofilm in some situations but may turn biofilm producers in other situations due to change in environmental factors5,6,7. This study was planned to test the hypothesis that non biofilm producer clinical isolates of staphylococci turn biofilm producers in presence of sodium chloride (isotonic) and high concentration of glucose, irrespective of presence or absence of ica operon.
Material & Methods
Isolation and identification of staphylococci was done as reported in our previous study8. The isolates were grouped in three categories: (i) Invasive isolates (100): Isolates obtained from two consecutive blood cultures of same patient. (ii) Colonizing isolates (50): Isolates from peripheral intravenous device (IVD) of the patients whose blood culture was negative for Staphylococcus. (iii) Commensal isolates (50): Staphylococcal isolates from skin and or nasal swab of patients, whose blood culture and peripheral IVD culture were negative for Staphylococcus.
Detection of biofilm: Isolates of S. aureus and coagulase negative staphylococci (CNS) were studied for biofilm producing capacity by microtiter plate method9 at 37° C, aerobically in the following culture media; A= Trypticase soy broth (TSB) alone, B= TSB+5 per cent glucose, C= TSB+10 per cent glucose, D= TSB+0.9 per cent NaCl, E= TSB+5 per cent glucose+0.9 per cent NaCl, F= TSB+10 per cent glucose+0.9 per cent NaCl.
Detection of ica ABDC genes: DNA from four to five colonies of each isolate was extracted10 and amplification of ica ADBC gene was done by using the protocol of Zeibhur et al11.
Statistical analysis: Statistical analysis was done using SPSS (Statistical Package for Social Scientists) software of 15.0 version, USA. Chi square test was used for comparison of proportion.
Results & Discussion
Of the 200 isolates, 139 were S. aureus and 61 were CNS. Of the 100 invasive isolates, 84 were S. aureus and 16 were CNS (3 S. epidermidis, 13 S. haemolyticus); of the 50 colonizing isolates, 30 were S. aureus and 20 were CNS (17 S. epidermidis, 3 S. xylosus), and of the 50 commensal isolates, 25 were S. aureus and 25 were CNS (23 S. epidermidis, 2 S. saprophyticus).
Of the 84 invasive S. aureus isolates, 67 (79%) showed biofilm producing potential, followed by colonizing 73 per cent (22/30) and commensals 28 per cent (7/25) isolates (P<0.001 among groups). All three (100%) invasive S. epidermidis isolates were biofilm producers followed by colonizing [70.5% (12/17)] and commensal isolates [39.1% (9/23)]. Though all S. haemolyticus isolates were invasive, but only 30.7 per cent (4/13) were able to produce biofilm. S. saprophyticus and S. xylosus both were non biofilm producing.
Staphylococcus aureus:
Invasive isolates - The mean absorbance value in different culture conditions increased from 0.386±.05 (control) to 1.67±.20 (highest in presence of both glucose and NaCl). Of the 67 biofilm positive invasive isolates, 63 were ica positive (53 ica ADBC positive, 10 ica AD positive) (Table I). Of the 17 biofilm negative isolates, 15 turned biofilm positive (10 ica ADBC positive, 3 ica AD positive, and 2 ica negative) in presence of either glucose or sodium chloride or both. Two isolates remained biofilm negative (both ica negative) (Table II).
Table I.
ica operon in biofilm positive and negative staphylococcal isolates
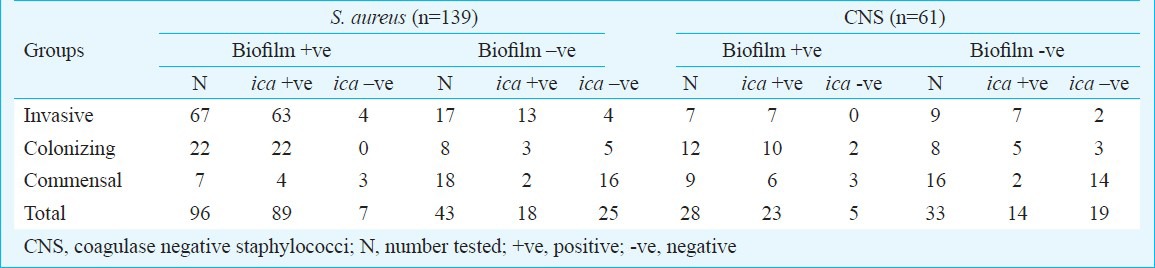
Table II.
Biofilm negative S. aureus and CNS isolates turned biofilm positive in different culture conditions
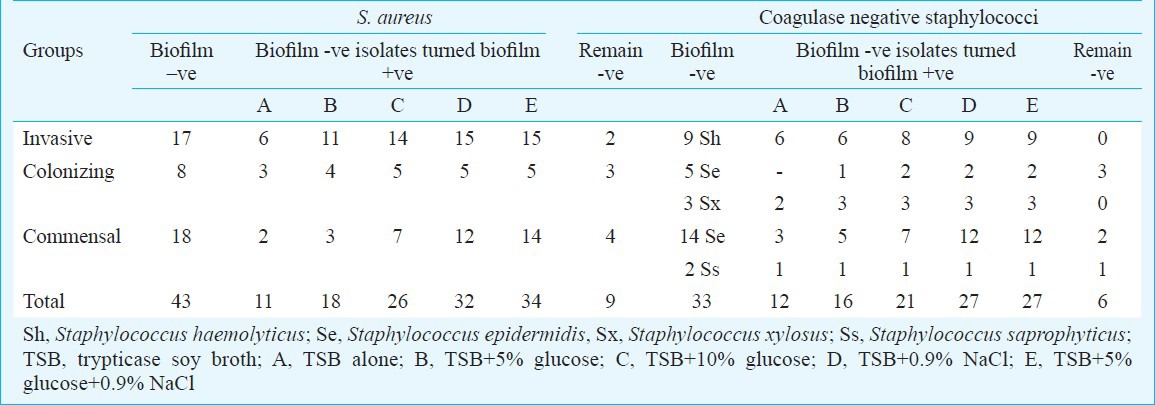
Colonizing isolates - The mean absorbance value in different culture conditions increased from 0.377±.02 (control) to 1.23±.10 (highest in presence of both glucose and NaCl). All 22 biofilm positive isolates were ica positive (15 ica ADBC positive, 7 ica AD positive) (Table I). Of the eight biofilm negative isolates, five turned biofilm positive (1 ica ADBC positive, 2 ica AD positive and 2 ica negative) in presence of glucose/ NaCl/ both, however, 3 remained biofilm negative (all ica negative) (Table II).
Commensal isolates - The mean absorbance value in different culture conditions increased from 0.276±.02 (control) to 1.23±.10 (highest in presence of both glucose and NaCl). Of the seven biofilm positive isolates, four were ica positive (2 ica ADBC positive, 2 ica AD positive) (Table I). Of the 18 biofilm negative isolates 14 turned biofilm positive (2 ica AD positive and 12 ica negative) and four remained biofilm negative (all ica negative) (Table II).
Coagulase negative staphylococci:
Invasive isolates - The mean absorbance value in different culture conditions increased from 0.353±.03 (control) to 1.46±.06 (highest in presence of both glucose and NaCl). All nine (S. haemolyticus) biofilm negative isolates turned biofilm positive (5 ica ADBC positive, 2 ica AD positive and 2 ica negative) in presence of glucose/NaCl/ both (Tables I, II).
Colonizing isolates - The mean absorbance value in different culture conditions increased from 0.453±.04 (control) to 1.33±.10 (highest in presence of both glucose and NaCl). Of the 12 S. epidermidis biofilm positive isolates, 10 were ica positive (7 ica ADBC positive, 3 ica AD positive) and two were ica negative (Table I). Five of eight biofilm negative isolates turned biofilm positive (one S. xylosus ica ADBC positive, one S. xylosus and 2 S. epidermidis ica AD positive and 1 S. epidermidis ica negative) in presence of glucose/NaCl/both but three isolates remained biofilm negative (Table II).
Commensal isolates - The mean absorbance value in different culture conditions increased from 0.301±.03 (control) to 0.755±.07 (highest in presence of both glucose and NaCl). Of the 9 biofilm positive S. epidermidis isolates, six were ica positive (4 ica ADBC positive, 2 ica AD positive). Of the 16 (12 S. epidermidis, 1 S. saprophyticus) biofilm negative isolates, 13 turned biofilm positive (1 S. epidermidis was ica ADBC positive, 1 S. Saprophyticus and 3 S. epidermidis were ica AD positive and 8 ica negative) in presence of glucose/NaCl/ both and three isolates remained biofilm negative (all ica negative) (Tables I, II).
The present study showed significant increase in biofilm production by clinical isolates of Staphylococcus when exposed to increasing concentration of glucose and sodium chloride. Increase was higher when NaCl and glucose both were supplemented in the medium. No significant biofilm formation has been reported if bacteria are grown in TSB alone irrespective of the fact that TSB already contains substantial amounts of glucose5. Supplementation of 0.2 per cent glucose in TSB is sufficient to induce a visible biofilm formation and a further increase in glucose concentration up to 1 per cent increases the biofilm formation significantly7,9,12. Rode et al6 compared individual effects of glucose and NaCl with the combination of glucose plus NaCl. Strains showed highest biofilm formation in the presence of glucose and NaCl rather than for each compound separately.
Expression of the ica ADBC operon is considered to be essential for the synthesis of polysaccharide intercellular adhesin (PIA), which mediates cell-to-cell adhesion1. Some biofilm producing isolates were found to be ica operon negative in our study, as has been reported earlier4. Cafiso et al13 analyzed the transcriptional activity of ica operon genes in a sample of biofilm positive and biofilm negative staphylococcal isolates and concluded that biofilm production takes place only when ica D was co-expressed with ica A. ica independent mechanism for biofilm formation was first studied by Cucarella et al14, who demonstrated that biofilm associated proteins (Bap) were involved in ica independent biofilm formation mechanism15. The production of PIA is subjected to on-off switching, and may be involved in phase-variation that might improve bacterial survival and growth under changing environmental conditions in vivo16. Environmental regulation may play an important role in biomaterial related disease. icaR is a strong negative regulator of the ica locus, as deletion of icaR augmented PIA production by nearly 10-fold and increased transcription of the ica locus by 100-fold17. The expression of this gene alone induces low enzymatic activity and the production of low amounts of polysaccharide. However, the simultaneous expression of icaA and icaD promotes a significant increase in N-acetylglucosaminyl transferase, with a consequent increase in the amount of polysaccharide, forming oligomers of 10-20 β-1,6-N-acetylglucosamine residues17.
Several ica independent regulatory genes have also been reported. The agr gene has been shown to increase biofilm detachment and mutation of the system, leading to decreased biofilm growth18,19. Staphylococcal accessory regulator (Sar) gene regulates genes of cell wall-associated adherence factors increasing the ica transcription and PIA/poly-N-acetyl glucosamine (PNAG) production20,21. Additional components, such as accumulation-associated protein (Aap), DNA and RNA independently or in cooperation with the ica operon, have also been suggested to be important in CNS biofilms22,23,24. Bap has been shown to be involved in the initial attachment, intercellular adhesion and biofilm formation of S. aureus14. NaCl has been found to significantly induce more biofilm in methicillin susceptible Staphylococcus aureus (MSSA) than in methicillin resistant Staphylococcus aureus (MRSA) isolates25. NaCl is a known activator of ica transcription26,27 and biofilm development in MSSA is ica ADBC dependent. Biofilm development in other isolates is primarily glucose induced, may be ica independent and involves a protein adhesion28.
In conclusion, our findings show that increasing the concentration of glucose and sodium chloride enhances biofilm production capacity of pathogenic as well as non pathogenic staphylococcal isolates irrespective of presence or absence of ica operon.
Acknowledgment
The work was supported by a grant from Indian Council of Medical Research, New Delhi.
References
- 1.Cramton SE, Gerke C, Schnell NF, Nicholas WW, Gotz F. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun. 1999;67:5427–33. doi: 10.1128/iai.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Götz F. Staphylococcus and biofilms. Mol Microbiol. 2002;43:1367–78. doi: 10.1046/j.1365-2958.2002.02827.x. [DOI] [PubMed] [Google Scholar]
- 3.O’Gara JP. ica and beyond: biofilm mechanisms and regulations in Stahylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol. 2007;270:179–88. doi: 10.1111/j.1574-6968.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- 4.Ninin E, Caroff N, Espaze E, Maraillac J, Lepelletier D, Milpied N, et al. Assessment of ica operon carriage and biofilm production in Staphylococcus epidermidis isolates causing bacteraemia in bone marrow transplant recipients. Clin Microbiol Infect. 2006;12:446–52. doi: 10.1111/j.1469-0691.2006.01382.x. [DOI] [PubMed] [Google Scholar]
- 5.Lim Y, Jana M, Luong TT, Lee YC. Control of glucose and NaCl induced biofilm formation by rbf in Staphylococcus aureus. J Bacteriol. 2004;186:722–9. doi: 10.1128/JB.186.3.722-729.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rode MT, Langsrud S, Holck A, Moretro T. Different patterns of biofilm formation in Staphylococcus aureus under food-related stress conditions. Int J Food Microbiol. 2007;116:372–83. doi: 10.1016/j.ijfoodmicro.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Seidl K, Goerke C, Wolz C, Mack D, Bachi BB, Bischoff M. Staphylococcus aureus Ccp affects biofilm formation. Infect Immun. 2008;76:2044–50. doi: 10.1128/IAI.00035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain A, Agarwal A. Biofilm production, a marker of pathogenic potential of colonizing and commensal staphylococci. J Microbiol Methods. 2009;76:88–92. doi: 10.1016/j.mimet.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Moretro T, Hermansen L, Askild L. Biofilm formation and the presence of the intercellular adhesion locus ica among staphylococci from food and food processing environments. Appl Environ Microbiol. 2003;69:5648–55. doi: 10.1128/AEM.69.9.5648-5655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryffel C, Kayser FH, Berger-Bachi B. Correlation between regulation of mecA transcription and expression of methicillin resistance in staphylococci. Antimicrob Agent Chemother. 1992;37:2952–61. doi: 10.1128/aac.36.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziebuhr W, Krimmer V, Rachid S, Lobner I, Gotz F, Hacker J. A novel mechanism of phase variation of virulence in Staphylococcus epidermidis: evidence for control of the polysaccharide intercellular adhesin synthesis by alternating insertion and excision of the insertion sequence element IS 256. Mol Microbiol. 1999;32:345–56. doi: 10.1046/j.1365-2958.1999.01353.x. [DOI] [PubMed] [Google Scholar]
- 12.Jahannes KM, Knobloch-Marthias A, Horstkotte HR, Mack D. Evaluation of different detection methods of biofilm formation in Staphylococcus aureus. Med Microbiol Immunol. 2002;191:101–6. doi: 10.1007/s00430-002-0124-3. [DOI] [PubMed] [Google Scholar]
- 13.Cafiso V, Bertuccio T, Santagati M, Campanile F, Amicosante G, Perilli MG, et al. Presence of the ica operon in clinical isolates of Staphylococcus epidermidis and its role in biofilm production. Clin Microbiol Infect. 2004;10:1081–8. doi: 10.1111/j.1469-0691.2004.01024.x. [DOI] [PubMed] [Google Scholar]
- 14.Cucarella C, Solano C, Valle J, Amorena B, Lasa I, Penadés JR. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J Bacteriol. 2001;183:2888–96. doi: 10.1128/JB.183.9.2888-2896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cucarella C, Tormo MA, Ubeda C. Role of biofilm associated protein Bap, in the pathogenesis of bovine Staphylococcus aureus. Infect Immun. 2004;72:2177–85. doi: 10.1128/IAI.72.4.2177-2185.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jefferson KK, Pier DB, Goldmann DA, Pier GB. The teicoplanin associated regulator (TcaR) and the intercellular adhesion locus regulator (IcaR) are transcriptional inhibitors of the ica locus in Staphylococcus aureus. J Bacteriol. 2004;186:2449–56. doi: 10.1128/JB.186.8.2449-2456.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan C, Burrows LL, Deber CM. Helix induction in antimicrobial peptides by alginate in biofilms. J Biol Chem. 2004;279:38749–54. doi: 10.1074/jbc.M406044200. [DOI] [PubMed] [Google Scholar]
- 18.Otto M. Quorum sensing control in Staphylococci - a target for antimicrobial drug therapy? FEMS Microbiol Lett. 2004;241:135–41. doi: 10.1016/j.femsle.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Valle J, Toledo-Arana A, Berasain C, Ghigo JM, Amorena B, Penad´es JR, et al. SarA and not sigmaB is essential for biofilm development by Staphylococcus aureus. Mol Microbiol. 2003;48:1075–87. doi: 10.1046/j.1365-2958.2003.03493.x. [DOI] [PubMed] [Google Scholar]
- 20.Conlon KM, Humphreys H, O’Gara JP. Inactivations of rsbU and sarA by IS256 represent novel mechanisms of biofilm phenotypic variation in Staphylococcus epidermidis. J Bacteriol. 2004;186:6208–19. doi: 10.1128/JB.186.18.6208-6219.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Silva GDI, Kantzanou M, Justice A. The ica operon and biofilm production in coagulase negative staphylococci associated with carriage and disease in a neonatal intensive care unit. J Clin Microbiol. 2002;40:382–8. doi: 10.1128/JCM.40.2.382-388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chokr A, Watier D, Eleaume H, Pangon B, Ghnassia JC, Mack D, et al. Correlation between biofilm formation and production of polysaccharide intercellular adhesion in clinical isolates of coagulase-negative staphylococci. Int J Med Microbiol. 2006;296:381–8. doi: 10.1016/j.ijmm.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 23.Jiang J, Sun JY, Ou YZ, Qin ZQ, Chen JM, Qu D. Influence of ica transcription on biofilm phenotype of Staphylococcus epidermidis clinical isolates. Shanghai Med J. 2006;29:40–4. [Google Scholar]
- 24.Fredheim EGA, Klingenberg C, Rohde H, Frankenberger S, Gaustad P, Flægstad T, et al. Biofilm formation by Staphylococcus haemolyticus. J Clin Microbiol. 2009;47:1172–80. doi: 10.1128/JCM.01891-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Neill JP, Humphreys H. Staphylococcus epidermidis biofilms: importance and implications. J Med Microbiol. 2001;50:582–7. doi: 10.1099/0022-1317-50-7-582. [DOI] [PubMed] [Google Scholar]
- 26.Fitzpatrick F, Humphreys H, O’Gara JP. The genetics of staphylococcal biofilm formation - will a greater understanding of pathogenesis lead to better management of device-related infection? Clin Microbiol Infect. 2005;11:967–73. doi: 10.1111/j.1469-0691.2005.01274.x. [DOI] [PubMed] [Google Scholar]
- 27.Rachid S, Ohlsen K, Wallner U, Hacker J, Hecker M, Ziebuhr W. Alternative transcription factor sigma (B) is involved in regulation of biofilm expression in a Staphylococcus aureus mucosal isolate. J Bacteriol. 2000;182:6824–6. doi: 10.1128/jb.182.23.6824-6826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Neill E, Pozzi C, Houston P, Smyth D, Humphreys H, Robinson DA, O’Gava JP. Association between methicillin susceptibility and biofilm regulation in Staphylococcus aureus isolates from device-related infections. J Clin Microbiol. 2007;45:1379–88. doi: 10.1128/JCM.02280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


