Abstract
Background:
The Korean version of Mini-Mental Status Examination (K-MMSE) and the Korean version of Addenbrooke Cognitive Examination (K-ACE) have been validated as quick neuropsychological tests for screening dementia in various clinical settings. Medial temporal atrophy (MTA) is an early pathological characteristic of Alzheimer's disease (AD). We aimed to assess the diagnostic validity of the fusion of the neuropsychological test and visual rating scale (VRS) of MTA in AD.
Materials and Methods:
A total of fifty subjects (25 AD, 25 controls) were included. The neuropsychological tests used were the K-MMSE and the K-ACE. T1 axial imaging visual rating scale (VRS) was applied for assessing the grade of MTA. We calculated the fusion score with the difference of neuropsychological test and VRS of MTA. The receiver operating characteristics (ROC) curve was used to determine optimal cut-off score, sensitivity and specificity of the fusion scores in screening AD.
Results:
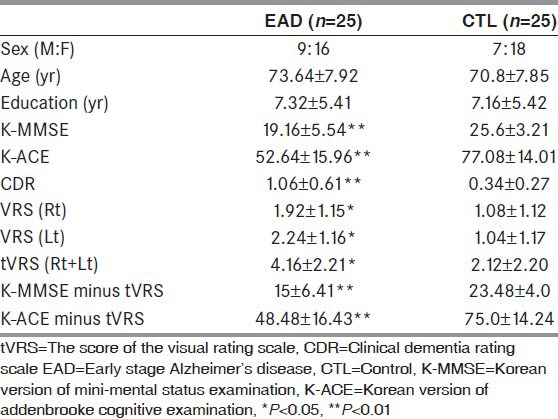
No significant differences in age, gender and education were found between AD and control group. The values of K-MMSE, K-ACE, CDR, VRS and cognitive function test minus VRS were significantly lower in the AD group than control group. The AUC (Area under the curve), sensitivity and specificity for K-MMSE minus VRS were 0.857, 84% and 80% and for K-ACE minus VRS were 0.884, 80% and 88%, respectively. Those for K-MMSE only were 0.842, 76% and 72% and for K-ACE only were 0.868, 80% and 88%, respectively.
Conclusions:
The fusion of the neuropsychological test and VRS suggested clinical usefulness in their easy and superiority over neuropsychological test only. However, this study failed to find any difference. This may be because of small numbers in the study or because there is no true difference.
Keywords: Alzheimer's disease, neuropsychological test, visual rating scale
Introduction
The Korean version of Mini-Mental Status Examination (K-MMSE) and the Korean version of Addenbrooke Cognitive Examination (K-ACE) have been used as quick and simple neuropsychological tests to screen for dementia. However, these screening neuropsychological tests have limited sensitivity and specificity to diagnose Alzheimer's disease (AD). The aims of this study is to assess the diagnostic validity of the fusion of the neuropsychological tests (K-MMSE or K-ACE) and T1-axial visual rating scale (VRS) of medial temporal lobe atrophy (MTA) in AD.
Background
Dementia patients are rapidly increasing as populations age worldwide.[1] As Korea is affected by this trend, the early diagnosis, treatment and prevention of dementia are conducted via the establishment of a dementia support center to minimize socioeconomic burdens of an increasing dementia population.[2] Various methods of neuropsychological assessment have been used to diagnose dementia, among which Mini-Mental Status Examination (MMSE) is the most widely used as a AD screening test. The MMSE is quick and can be applied in a short time. However, as shown in a recent meta-analysis, it has intermediate accuracy as its sensitivity and specificity are 81% and 87%, respectively.[3] According to a recent domestic study by Oh, the K-MMSE was reported to have sensitivity 80% and specificity 70%, even lower than the previous result.[4] To overcome the disadvantages of the MMSE, ACE in which the evaluation of memory, language and visuospatial and verbal fluency are added to the MMSE has been developed and used in many countries including Korea.[5]
Atrophy of the hippocampus and medial temporal lobe involved in memory function is as a characteristic neuropathological change in the early stage of AD.[6,7] Definite atrophy of the medial temporal lobe is observed in patients with mild cognitive impairment had a high risk of AD and dementia patients with more severe medial temporal atrophy showed more severe memory impairment on MRI.[8,9] In addition, medial temporal atrophy is closely associated with post-stroke amnestic cognitive impairment.[10] Currently, Schelten T1-coronal visual rating scale is the most commonly used visual rating scale that assesses the severity of medial temporal atrophy. It evaluates medial temporal atrophy using T1-coronal images obtained in an interval of 5 mm to the observation direction.[11] However, T1-coronal images may not be available in medical centers conducting MRI or the angle of the reference axis may vary depending on medical centers. Thus, it has disadvantages of requiring constant images from patients in a large-scale clinical study, and of difficulty in assessing medial temporal atrophy if evaluators are unfamiliar with the anatomical structure of the hippocampus.[12] To overcome these disadvantages, Kim suggested a visual rating scale that can easily measure the severity of medial temporal atrophy in AD patients using T1-axial images included in brain MRI.[12]
Accordingly, this study was conducted to investigate if more accurate diagnosis of early AD is possible when neuropsychological tests such as MMSE or ACE and VRS that reflects the anatomical and pathological changes of the brain, are simultaneously performed.
Materials and Methods
Subjects
This study was conducted on fifty patients aged 60-85 years who visited the department of neurology at Seoul Medical Center. AD was defined as a state that satisfies the diagnostic criteria of probable AD described in the Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA).[13] Among the subjects, those who did not meet the diagnostic criteria of AD and mild cognitive impairment and who were judged to have normal cognitive function in neurological tests were selected as the control group.
Methods
Neuropsychological tests
Seoul Neuropsychological Screening Battery (SNSB) was performed to evaluate detailed cognitive functions required for diagnosis.[14] All tests were conducted by one experienced psychologist. SNSB consisted of K-MMSE test, attention tests, lingual and relevant tests, visuospatial test, memory function items, frontal/executive function items, Geriatric Depression Scale (GDS), Barthel Activities of Daily Living (B-ADL) and Clinical Dementia Rating Scale (CDR). In this study, the data included in the SNSB was used for the K-MMSE. The K-ACE, which has higher specificity and sensitivity than those of the K-MMSE, was additionally used.[5]
Brain MRI imaging and visual rating scale
A radiologic test using 1.5 Tesla MRI (SIEMENS, Avanto Syngo) was conducted on all the patients. The images were fast spin echo T1-weighted images and 5 mm thickness axial images that were parallel to the line connecting the anterior commissure and posterior commissure were obtained. The TR, TE and flip angle were shown to be 500 msec, 11 msec and 90°, respectively.
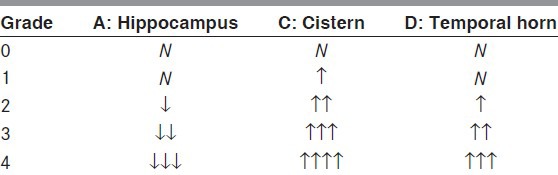
T1 axial imaging visual rating scale, which has been already proven to have a high correlation with Schelten coronal visual rating scale, was used.[12] In summary, using the images showing the midbrain where the hippocampus is the most clearly observed, the longest width of the hippocampus (A), The shortest distance between the hippocampus and brainstem, that is, the width of the crural cistern and ambient cistern (B) and the width of the temporal horn of lateral ventricle (C) were assessed and scored from 0-4 points. Both left and right sides were assessed [Table 1].[12] In this study, the score of the visual rating scale (tVRS) was obtained by summing the left and right scores.
Table 1.
Axial visual rating scale (Kim et al., 2009)
The fusion score of neuropsychological test and visual rating scale
The fusion score of the neuropsychological test and visual rating scale were calculated with the difference of the tests (e.g., K-MMSE -tVRS, K-ACE -tVRS).
Statistical analysis
Mann-Whitney U test was conducted to analyze the mean age, education and K-MMSE and K-ACE scores of the AD patients and control group, respectively. Chi-square test and Fisher's exact test were conducted for gender comparison. Statistical analysis was conducted using SPSS (SPSS V11.5K, SPSS Inc., Chicago, IL, USA). Null hypotheses of no difference were rejected if P values were less than 0.05. Receiver operation characteristics (ROC) curve analysis was conducted for the comparison between cognitive function tests and integrated tests (cognitive function test and visual rating scale), the specificity and sensitivity of the tests and the optimal cutoff point, followed by statistical analysis using SPSS 11.5 version. The statistical significance of the area under curve (AUC) among the cognitive function tests was compared using MedCalc 11.4 version.
Results
General characteristics of the subjects
The sociodemographic characteristics and mean value of neuropsychological tests of the subjects are presented in Table 2. A total of firty subjects were recruited and they consisted of 25 AD patients and 25 control patients. No significant difference in age, gender ratio and education was found between the two groups. Neuropsychological tests showed that the values of K-MMSE, K-ACE, CDR, VRS and cognitive function test -VRS were significantly lower in the AD group than in the control group.
Table 2.
Baseline characteristics of the subjects
Cognitive function test and VRS
In the validity of each test in the diagnosis of AD, the AUC of the K-ACE -tVRS was shown to be 0.884, which was the highest [Figure 1]. The AUC of the K-ACE, K-MMSE -tVRS and K-MMSE were shown to be 0.868, 0.857 and 0.842, respectively. The optimal cut-off score, sensitivity and specificity of each test were shown to be 67/68, 80% and 88%, respectively, for K-ACE -tVRS, 68/69, 80% and 88%, respectively, for K-ACE, 20/21, 84% and 80%, respectively, for K-MMSE -tVRS and 23/24, 76% and 72%, respectively, for K-MMSE [Table 3]. The AUC was shown to be higher in the simultaneous conduct of the K-ACE and VRS than in the conduct of K-ACE alone, but no significant difference was found when it was statistically analyzed using MedCalc. The same result was obtained in the comparison between the simultaneous conduct of the K-MMSE and tVRS and the conduct of the K-MMSE alone (P = 0.630 for K-MMSE - tVRS vs. K-MMSE).
Figure 1.
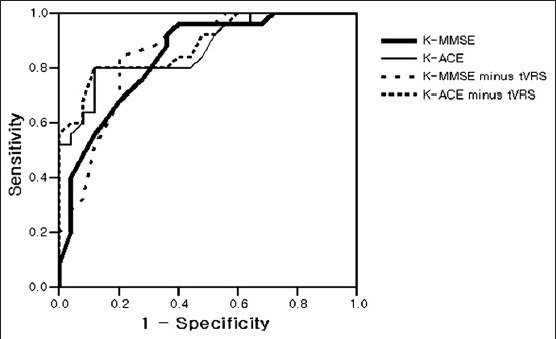
Comparison of ROC curves of neuropsychological tests and visual rating scale for AD
Table 3.
Optimal cut-off scores of the four neuropsychological tests and visual rating scale for detecting AD

Discussion
In this study, changes in the reliability and validity of AD screening were investigated when the assessment of medial temporal atrophy was additionally added to the K-MMSE and K-ACE. For the measurement of medial temporal atrophy, a visual rating scale using T1axial view images that are the most commonly obtained from MRI was used considering future clinical usefulness. In the diagnosis of AD, the AUC, specificity and sensitivity were shown to be higher in the addition of medial temporal atrophy visual rating scale to the K-MMSE or K-ACE than in the conduct of K-MMSE or K-ACE alone. However, no significant difference was found when the AUC was compared among the tests. Thus, it was concluded that the addition of medial temporal atrophy visual rating scale to either K-MMSE or K-ACE was not more useful for screening than K-MMSE or K-ACE alone. However, considering that this was a preliminary study and that the AUC, specificity and sensitivity increased when the VRS was added to the K-MMSE or K-ACE, it can not be ruled out that better results might be obtained from a further study. In addition, though the method of the hippocampal volume calculation is labor intensive and complex, the sum of hippocampal volume and MMSE showed usefulness in the diagnosis of mild cognitive impairment patients.[15]
Many pathologic studies reported that AD pathology was associated with MTA. MTA, which is observed on MRI in AD patients, has been known to be associated with Neurofibrillary tangle (NFT) pathology.[16] In addition, Csernansky reported that the density of NFT in the cornu ammonis 1 region of the hippocampus (CA1) was associated with hippocampal volume (reference). Zarow reported that the neuronal loss and volume reduction of CA1 were correlated with dementia severity.[17,18] However, in a postmortem autopsy study conducted on twenty three patients with Lewy body dementia and 8 AD patients, no significant relation was found between amyloid plaque and NFT pathological burden. MTA has been known to be not always caused by AD pathology.[19]
The clinical usefulness of MTA is still open to question. In a study that used particular imaging analysis methods such as voxel based morphometry, the screening and diagnosis of AD and frontotemporal dementia did not differ. As the aforementioned methods have disadvantages of requiring significant time and complex programs to be applied to actual clinical practices, the clinical application of MTA is limited.[20] However, many previous clinical studies reported that MTA was important for distinguishing early AD patients from normal or patients with mild cognitive impairment.[21,22,23] In addition, with respect to the usefulness of visual rating scale, Burton reported that MTA on MRI, which was measured using VRS, had robust discriminatory power for screening and diagnosing AD, Lewy body dementia and vascular cognitive impairment in dementia patients who were pathologically verified and that its sensitivity and specificity were 91% and 94%, respectively.[16] Recently, Westman also reported that when MTA via VRS was compared with computerized multivariate classification and manual hippocampal volume measurements, a similar degree of accuracy was shown in the diagnosis of AD (81% for the visual rating assessments, 83% for multivariate classification, 89% for manual measurements of total hippocampal volume).[24]
The result of this study showed that the simultaneous conduct of cognitive function test, which is a general AD screening test and VRS was not superior to the sole conduct of cognitive function test. Though the previous combining study using hippocampal volume and MMSE with similar sample size (eighteen patients, seventeen control) showed statistical significance, the subject number of fifty in this study was insufficient for assessing the usefulness of the tests, which could have reduced statistical accuracy.[15] Furthermore, the patients of the control group who visited the department of neurology are likely to have subjective memory impairment. These patients could affect the result of the test although no significant correlation between subjective memory impairment and objective cognitive functions was reported in a short-term cross-sectional study.[25] If a further large-scale study is conducted based on the results of this preliminary study, a more efficient AD screening method could be developed and the assessment of MTA using VRS could be helpful for the rapid and accurate diagnosis of AD in clinical practices.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Alzheimer's Disease Internationa: Global prevalence of dementia: A Delphi consensus study. Lancet. 2005;366:2112–7. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heo JH, Kim HS, Bae-HJ, Lee K, Bae MH, Lee JB. Trend in treatment of dementia by benefit cost status based on health insurance review and assessment service (HIRA)'s data from 2003 to 2007. Dement Neurocognitive Disord. 2010;9:135–9. [Google Scholar]
- 3.Mitchell AJ. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res. 2009;43:411–31. doi: 10.1016/j.jpsychires.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Oh E, Kang YW, Shin JH, Yeon BK. A validity study of K-MMSE as a screening test for dementia: Comparison against a comprehensive neuropsychological evaluation. Dement Neurocognitive Disord. 2010;9:8–12. [Google Scholar]
- 5.Heo JH, Lee KM, Park TH, Ahn JY, Kim MK. Validation of the Korean Addenbrooke's cognitive examination for diagnosing Alzheimer's dementia and mild cognitive impairment in the Korea elderly. Appl Neuropsychol. 2012;19:127–31. doi: 10.1080/09084282.2011.643948. [DOI] [PubMed] [Google Scholar]
- 6.Barkhof F, Polvikoski TM, van Straaten EC, Kalaria RN, Sulkava R, Aronen HJ, et al. The significance of medial temporal lobe atrophy: A postmortem MRI study in the very old. Neurology. 2007;69:1521–7. doi: 10.1212/01.wnl.0000277459.83543.99. [DOI] [PubMed] [Google Scholar]
- 7.Mistur R, Mosconi L, Santi SD, Guzman M, Li Y, Tsui W, et al. Current challenges for the early detection of Alzheimer's disease: Brain imaging and CSF studies. J Clin Neurol. 2009;5:153–66. doi: 10.3988/jcn.2009.5.4.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rusinek H, Endo Y, De Santi S, Frid D, Tsui WH, Segal S, et al. Atrophy rate in medial temporal lobe during progression of Alzheimer disease. Neurology. 2004;63:2354–9. doi: 10.1212/01.wnl.0000148602.30175.ac. [DOI] [PubMed] [Google Scholar]
- 9.Visser PJ, Verhey FR, Hofman PA, Scheltens P, Jolles J. Medial temporal lobe atrophy predicts Alzheimer's disease in patients with minor cognitive impairment. J Neurol Neurosurg Psychiatry. 2002;72:491–7. doi: 10.1136/jnnp.72.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim BJ, Oh MY, Jang MS, Han MK, Lee J, Lee J, et al. Medial temporal atrophy and memory dysfunction in poststroke cognitive impairment-no dementia. J Clin Neurol. 2012;8:43–50. doi: 10.3988/jcn.2012.8.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, et al. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer's disease and normal ageing: Diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55:967–72. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim GH, Kwon HJ, Go SA, Kim JE, Park KD, Ghoi KG, et al. T1-Axial Medial Temporal Atrophy Visual Rating: A Comparable Study with Schelten's T1-Coronal Visual Rating. Dement Neurocognitive Disord. 2009;8:37–44. [Google Scholar]
- 13.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 14.Kang YW, Na DL. Seoul: Human Brain Research and Consulting Co; 2003. Seoul Neuropsychological Screening Battery. [Google Scholar]
- 15.Slavin MJ, Sandstrom CK, Tran TT, Doraiswamy PM, Retrella JR. Hippocampal volume and the mini-mental state examination in the diagnosis of amnestic mild cognitive impairment. AJR Am J Roentgenol. 2007;188:1404–10. doi: 10.2214/AJR.06.1052. [DOI] [PubMed] [Google Scholar]
- 16.Burton EJ, Barber R, Mukaetova-Ladinska EB, Robson J, Perry RH, Jaros E, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer's disease from dementia with Lewy bodies and vascular cognitive impairment: A prospective study with pathological verification of diagnosis. Brain. 2009;132:195–203. doi: 10.1093/brain/awn298. [DOI] [PubMed] [Google Scholar]
- 17.Csernansky JG, Hamstra J, Wang L, McKeel D, Price JL, Gado M, et al. Correlations between antemortem hippocampal volume and postmortem neuropathology in AD subjects. Alzheimer Dis Assoc Disord. 2004;18:190–5. [PubMed] [Google Scholar]
- 18.Zarrow C, Vinters HV, Ellis WG, Weiner MW, Mungas D, White L, et al. Correlates of hippocampal neuron number in Alzheimer's disease and ischemic vascular dementia. Ann Neurol. 2005;57:896–903. doi: 10.1002/ana.20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burton EJ, Mukaetova-Ladinska EB, Perry RH, Jaros E, Barber R, O’Brien JT, et al. Quantitative neurodegenerative pathology does not explain the degree of hippocampal atrophy on MRI in degenerative dementia. Int J Geriatr Psychiatry. 2012;27:1267–74. doi: 10.1002/gps.3774. [DOI] [PubMed] [Google Scholar]
- 20.Galton CJ, Gomez-Anson B, Antoun N, Scheltens P, Patterson K, Graves M, et al. Temporal lobe rating scale: Application to Alzheimer's disease and frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2001;70:165–73. doi: 10.1136/jnnp.70.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryu SY, Kwon MJ, Lee SB, Yang DW, Kim TW, Song IU, et al. Measurement of precuneal and hippocampal volumes using magnetic resonance volumetry in Alzheimer's disease. J Clin Neurol. 2010;6:196–203. doi: 10.3988/jcn.2010.6.4.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeCarli C, Frisoni GB, Clark CM, Harvey D, Grundman M, Petersen RC, et al. Qualitative estimates of medial temporal atrophy as a predictor of progression from mild cognitive impairment to dementia. Arch Neurol. 2007;64:108–15. doi: 10.1001/archneur.64.1.108. [DOI] [PubMed] [Google Scholar]
- 23.Korf ES, Wahlund LO, Visser PJ, Scheltens P. Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurology. 2004;63:94–100. doi: 10.1212/01.wnl.0000133114.92694.93. [DOI] [PubMed] [Google Scholar]
- 24.Westman E, Cavallin L, Muehlboeck JS, Zhang Y, Mecocci P, Vellas B, et al. Sensitivity and specificity of medial temporal lobe visual ratings and multivariate regional MRI classification in Alzheimer's disease. PLoS One. 2011;6:22506. doi: 10.1371/journal.pone.0022506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chin J, Oh KJ, Seo SW, Shin HY, Na DL. The characteristics and subtypes of subjective memory impairment in older adults. Dement Neurocognitive Disord. 2010;9:115–21. [Google Scholar]


