Figure 2. Tat–beclin 1 peptide binds to GAPR-1, a beclin 1-interacting protein.
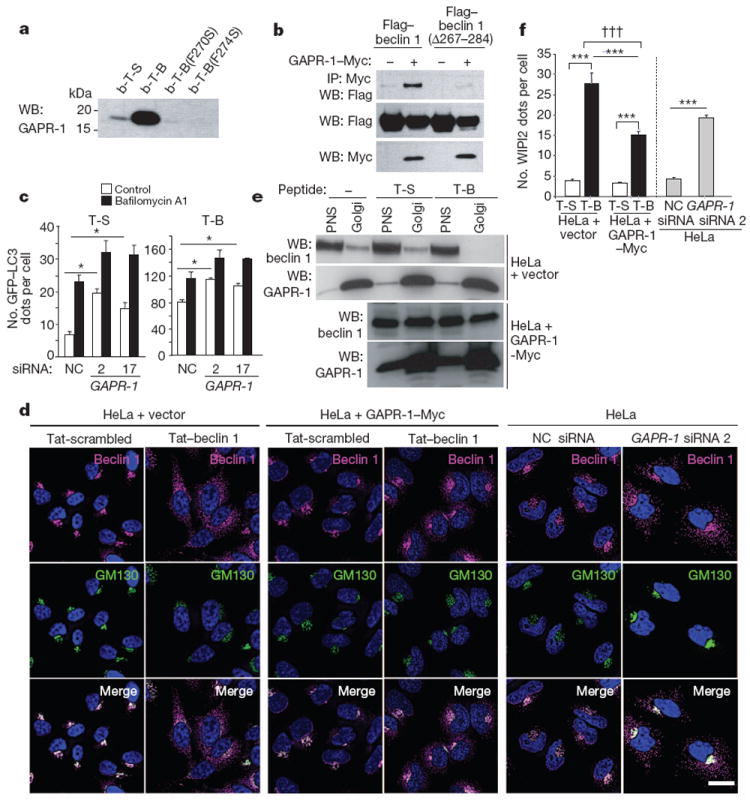
a, HeLa cells were treated with biotin-conjugated peptides (30 μM, 3 h) and proteins bound to peptides were analysed by immunoblot with anti-GAPR-1. b-T-B, biotin–Tat–beclin 1; b-T-S, biotin–Tat-scrambled. b, Immunoprecipitation of Flag–beclin 1 with GAPR-1–Myc in HeLa cells 24 h post-transfection. c, GFP–LC3-positive dots in GAPR-1 siRNA-transfected peptide-treated HeLa/GFP–LC3 cells (20 μM, 3 h) with or without 100nM bafilomycin A1. Bars represent mean ± s.e.m. of triplicate samples (50–100 cells per sample). Similar results were observed in three independent experiments. NC, non-silencing control. d, Localization of beclin 1 and GM130 (a Golgi marker) in HeLa cells stably transduced with empty vector (left panel) or GAPR-1–Myc (middle panel) or transfected with GAPR-1 siRNA (right panel) and treated with peptide (20 μM, 1 h). Scale bar, 20 μm. e, Immunoblot of beclin 1 in post-nuclear supernatant (PNS) and Golgi-enriched fractions in HeLa cells stably transduced with empty vector or GAPR-1–Myc after peptide treatment (20 μM, 2 h). f, WIPI2 dots in cells in the experimental conditions shown in d. Bars represent mean ± s.e.m. for 100–150 cells. *P < 0.05, ***P < 0.001; t-test. †††P < 0.001; two-way ANOVA for comparison of magnitude of changes between groups.
