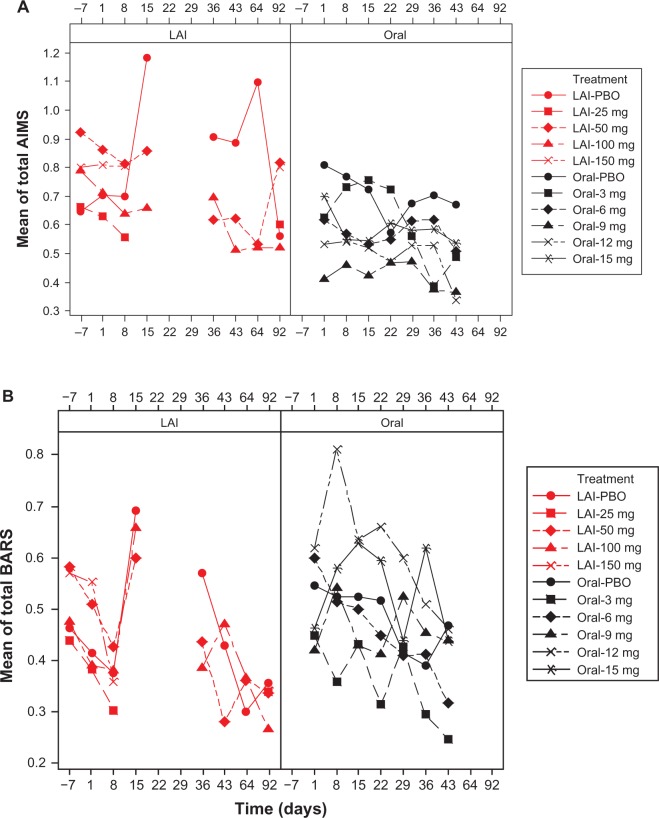
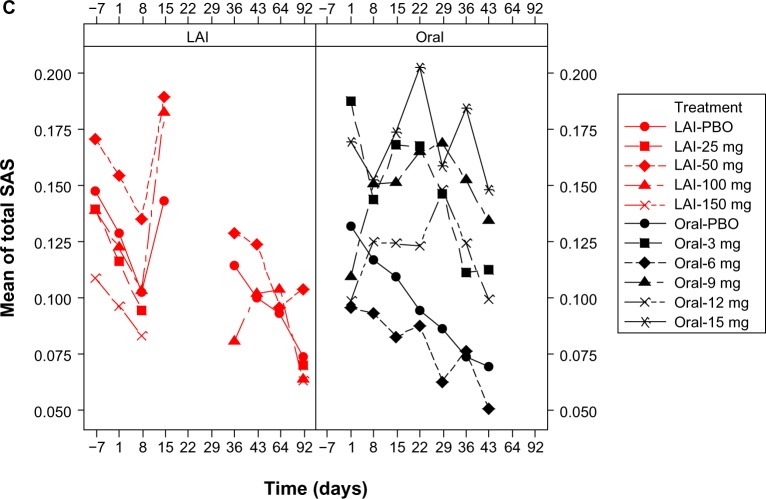
Figure 1.
Mean EPS total scores over time by dose: pooled LAI versus oral paliperidone (safety analysis set). (A) Mean AIMS total scores, (B) mean BARS total scores, and (C) mean SAS total scores.
Notes: Doses of paliperidone-LAI shown are in mg eq.. Number of patients in each treatment (by dose) group changed over time. One LAI study, which included treatment groups of LAI placebo, LAI 50 mg eq., and LAI 100 mg eq., had AIMS, BARS, and SAS measured at days −7, 1, 8, 15, 36, 43, 64, and 92. The rest of the three LAI studies, which when combined included treatment groups of LAI placebo, LAI 25 mg eq., LAI 50 mg eq., LAI 100 mg eq., and LAI 150 mg eq., had AIMS, BARS, and SAS measured at days −7, 1, 8, and 92. Three oral studies, which when combined included treatment groups of oral placebo, 3 mg, 6 mg, 9 mg, 12 mg, and 15 mg, had AIMS, BARS, and SAS measured at days 1, 8, 15, 22, 29, 36, and 43. LAI results for time points from days 15 to 64 are only from one study; data were not collected on day 22 and 29 in any of the LAI studies; hence the gaps in graphs.
Abbreviations: AIMS, Abnormal Involuntary Movement Scale; BARS, Barnes Akathisia Rating Scale; LAI, long-acting injectable; LAI-PBO, placebo group in LAI studies; mg eq, milligram equivalent; Oral-PBO, placebo group in oral studies; SAS, Simpson Angus Scale.