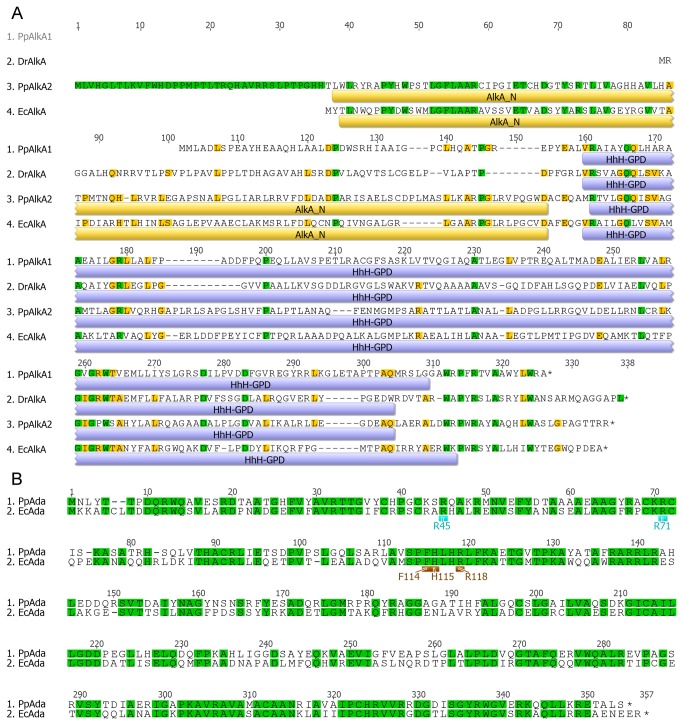
Figure 2. The multiple protein alignment of E. coli K-12 DH10B EcAlkA (locus tag: ECDH10B_2218), P. putida KT2440 PpAlkA (PP_0705), P. putida GB-1 PpAlkA2 (PputGB1_2545), and D. radiodurans DrAlkA (DR_2584) generated with ClustalW (A).
Yellow and blue bars represent Pfam domains corresponding to the above sequence: AlkA_N – the N-terminal domain of 3meA glycosylase AlkA proteins; HhH-GPD – helix-hairpin-helix domain with Gly/Pro rich loop followed by a conserved aspartate residue. The multiple protein alignment of P. putida KT2440 PpAda (PP_0706) and E. coli K-12 DH10B EcAda (ECDH10B_2370) proteins (B) shows the perfect conservation of residues responsible for the specific interaction with the conserved nucleotide sequences of the ada regulon promoters: R45 and R71 (light blue) binding to the A box and F114, H115, and R118 (brown) binding to the B box.