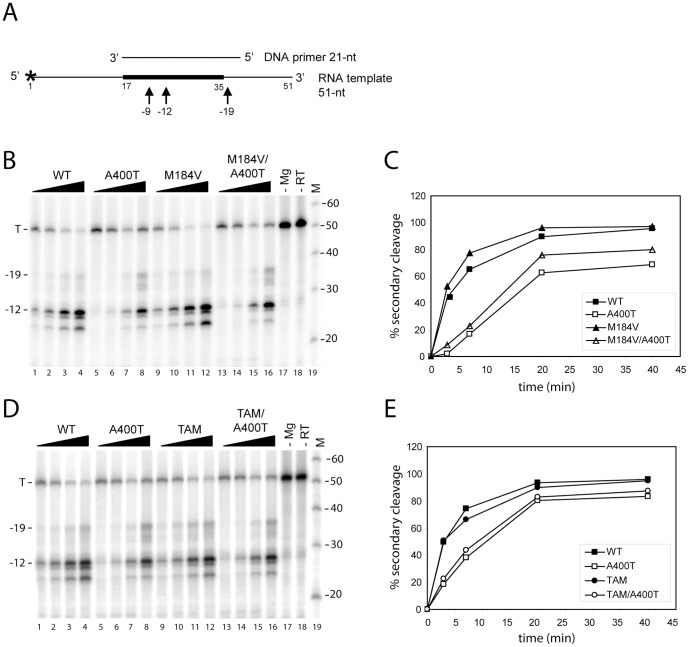
Figure 3. RNaseH activity of virus-derived RT enzymes containing substitution A400T.
(A) RNaseH cleavage was measured using a 5′-end labeled (asterisk) 51-nt HIV-1 RNA template representing the PBS region. A 21-nt DNA primer complementary to the PBS was heat-annealed onto the RNA template. Primary (−19) and secondary (−12/−9) cleavages were monitored. (B) Representative gel showing RNaseH cleavage mediated by the virion-derived RTs with substitution A400T in the wild-type and M184V background. The reactions were initiated by the addition of Mg2+, and template (T) cleavage was monitored after 3, 7, 20 and 40 min of incubation. Control reactions were performed in the absence of Mg2+ (lane 18) or RT (lane 19), showing no template degradation. (C) The formation of secondary cleavage products over time was quantified, and is plotted for this representative gel. (D) RNaseH cleavage mediated by the virion-derived RTs with substitution A400T in the wild-type and TAM background was analyzed, and formation of secondary cleavage products is plotted in (E). Representative experiments are shown, and three independent experiments were performed with similar results.