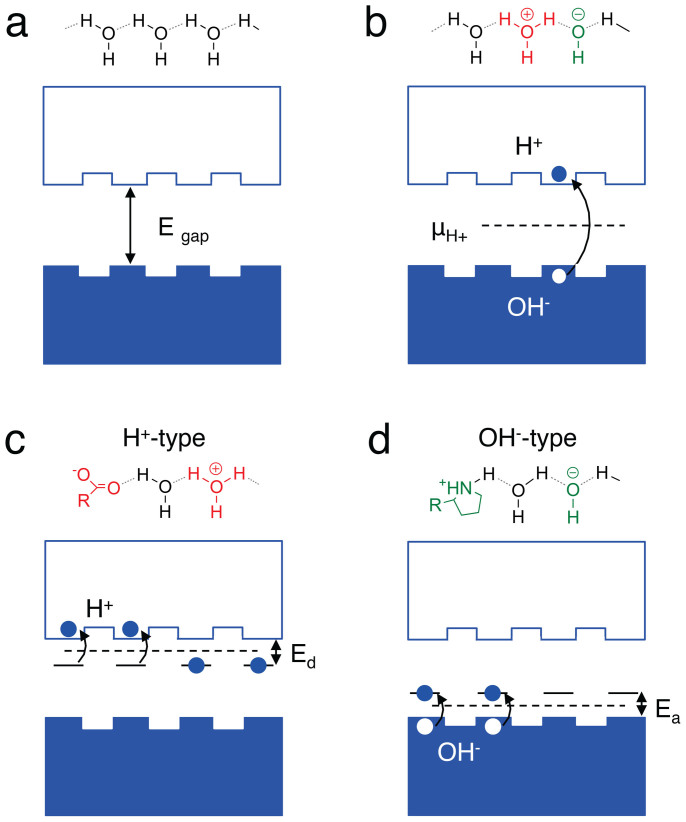
Figure 2. Energy diagram representation of conduction in hydrogen bonded proton wire.
(a) A wire with no H+ or OH− defect does not conduct. The band gap is defined as the energy required to create a H+ OH− pair (proton-proton hole) and is derived from the Egap = ΔG0′ = −kBT ln Kw = 0.83 eV (Gibbs-Helmholtz equation). (b) For an intrinsic proton wire, the protochemical potential uH+ is in the middle of the band-gap. The H+ is not completely delocalized along the conduction quasi band. Hopping barriers of approximately 100 meV (need to be overcome for conduction to occur. (c) An acid donates a H+ into the conduction band of a proton wire to yield a H+-type protonic conductor. Ed = ΔG0a = −kBT ln Ka, Ka is the acid dissociation constant. The maleic acid group pKa (-log Ka) = 3.2, which corresponds to Ed = 0.18 eV. (d) A base accepts a H+ to create a OH− (proton hole) in the valence band of a proton wire to yield a OH−-type protonic conductor. Ea = ΔG0b = −kBT ln Kb, Kb is the base dissociation constant. The proline base pkb (-log Kb = 3.4), which corresponds to Ea = 0.20 eV. For both H+ type and OH− type the protochemical potential is μCH+ = eV0 + µ0+ kBT ln aH+ where aH+ is the activity of H+.