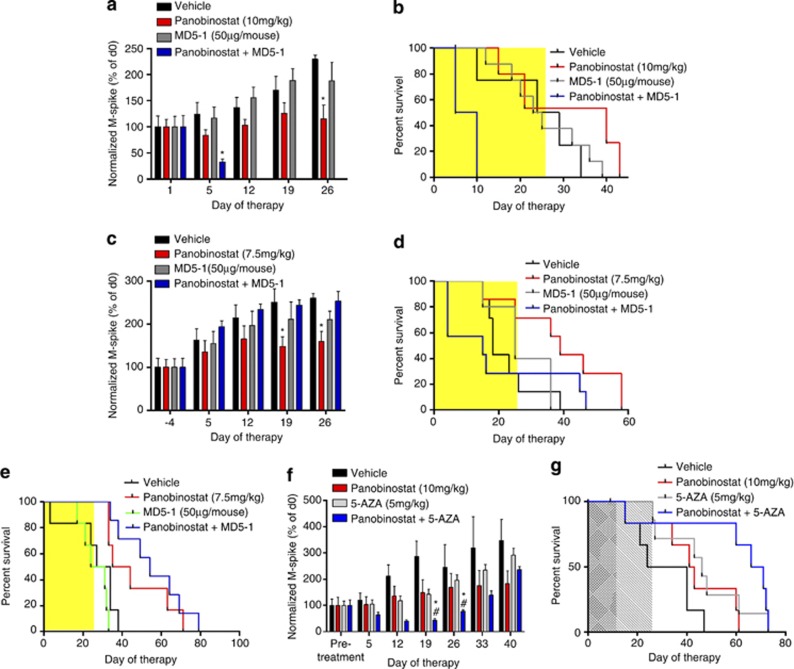
Figure 7.
In vivo treatment consisting of panobinostat in combination with MD5-1 is not well tolerated and does not enhance survival of C57BL/6 mice bearing Vk*MYC MM over single-agent panobinostat treatment alone, whereas its combination with 5-AZA provides significant benefit. (a) Normalized M-spike of mice bearing Vk*MYC MM treated as follows: vehicle (D5W±control antibody UC81B9, n=8); panobinostat (10 mg/kg, n=6); MD5-1 (50 μg per mouse; days 1, 4, 8, 12; n=8); or the combination of both agents (n=8). *P<0.05 versus vehicle. (b) Survival of mice treated as per 7A, (c) normalized M-spike of mice bearing Vk*MYC MM treated as follows: vehicle (D5W, n=7); panobinostat (7.5 mg/kg, n=7); MD5-1 (50 μg per mouse; days 2, 5, 9, 12; n=6); or the combination of both agents (n=7); (d) survival of mice treated as per (c); (e) absence of on-target MD5-1-mediated toxicity by treatment of C57BL/6.DR5 KO mice bearing Vk*MYC tumor with panobinostat and MD5-1 combination therapy leads to significant increases in survival. Mice were treated as follows: vehicle (D5W±control antibody UC81B9, n=6); panobinostat (7.5 mg/kg, n=6); MD5-1 (50μg per mouse, days 2, 5, 9, 12; n=6); or the combination of both agents (n=7); (f) normalized M-spike of mice bearing Vk*MYC MM treated as follows: vehicle (D5W, n=6), panobinostat (10 mg/kg, n=6), 5-AZA (5 mg/kg, n=7) and the combination of both agents (n=7). (g) Survival of mice treated as per (f). *P<0.05 versus vehicle and #P<0.05 versus initial (pretreatment) SPEP