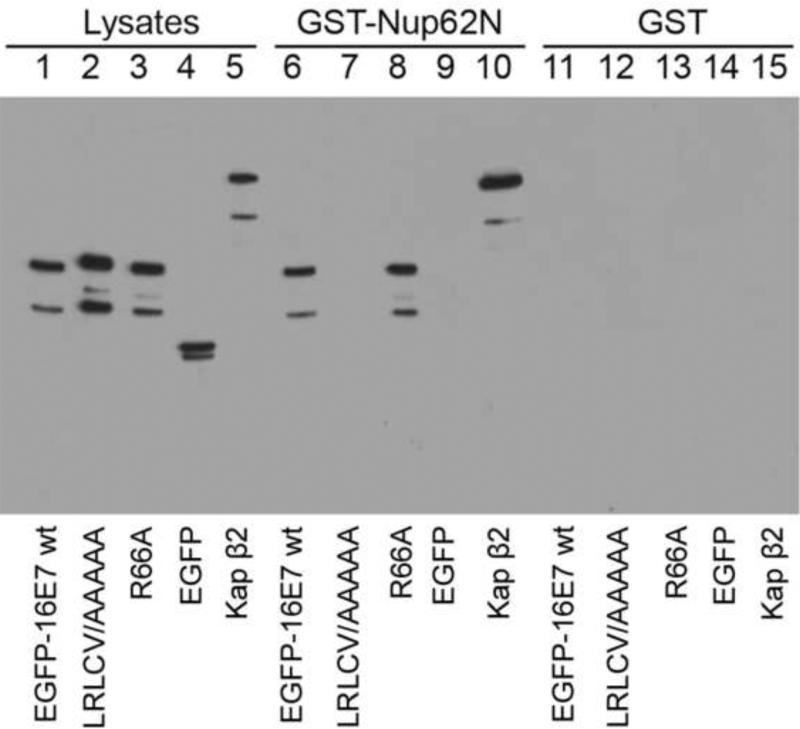
Fig. 7. HPV16 E7 interacts with the FG domain of Nup62 and mutations of hydrophobic residues within the zinc-binding domain disrupt this interaction.
HeLa cells were transfected with EGFP-16E7, EGFP-16E7LRLCV65AAAAA, EGFP-16E7R66A, and EGFP plasmids. Cell lysates were prepared 24 h post transfection and probed with either a GFP antibody (lane 1, EGFP-16E7; lane 2, EGFP-16E7LRLCV65AAAAA; lane 3, EGFP-16E7R66A; lane 4, EGFP) or Kap 2 antibody (lane 5). GST-Nup62N (lanes 6-10) and GST (lanes 11-15) immobilized on glutathione-Sepharose were incubated with the cell lysates and the bound proteins were eluted and analyzed via immunoblotting with a GFP antibody (lanes 6 and 11, EGFP-16E7; lanes 7 and 12, EGFP-16E7 LRLCV65AAAAA; lanes 8 and 13, EGFP-16E7R66A; and lanes 9 and 14, EGFP). Binding of Kap 2 to GSTNup62N and GST was detected with a Kap 2 antibody (lanes 10 and 15).