Figure 3. ReAsH Labeling of the JM Antiparallel Helices is Linked to a Global Active Conformation.
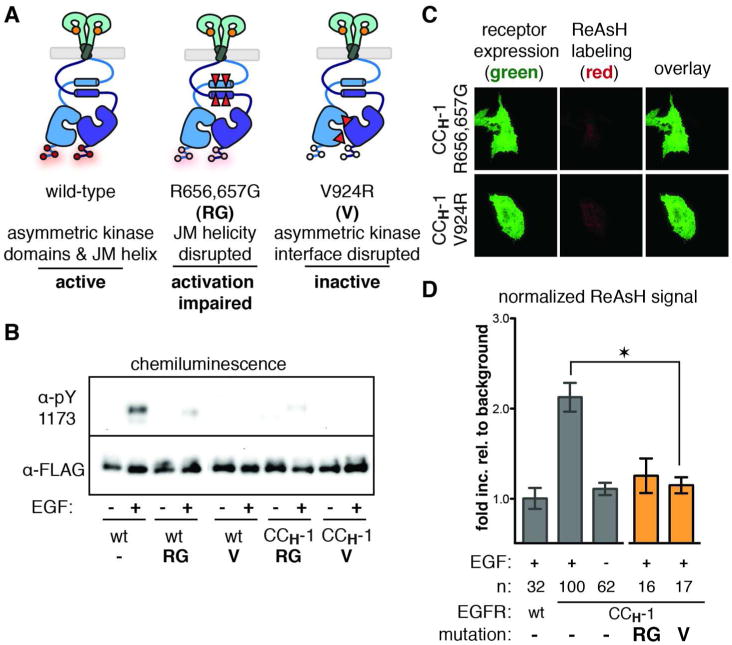
(a) Cartoon depicting the relative positions of the activation-impairing EGFR mutations R656,657G and V924R. (b) Western blots confirm that these mutants are defective in tyrosine autophosphorylation in the context of wild-type and CCH-1 EGFR. (c) Representative TIRFM images of ReAsH-treated cells expressing CCH-1 EGFR variants containing the R656,657G or V924R mutations. (d) Quantification of TIRFM results as a fold increase relative to background that is normalized for receptor expression levels. Error bars represent the standard error. * represents p<0.05 based on ANOVA with Bonferroni post-test.
The ability of CCH-1 to bind ReAsH is dependent on the presence of JM helices (R656,657G) and the global active conformation of kinase domains (V924R). The absence of ReAsH labeling in these variants provides a structural link between the receptor activation and formation of an antiparallel JM coiled coil.
