Figure 3.
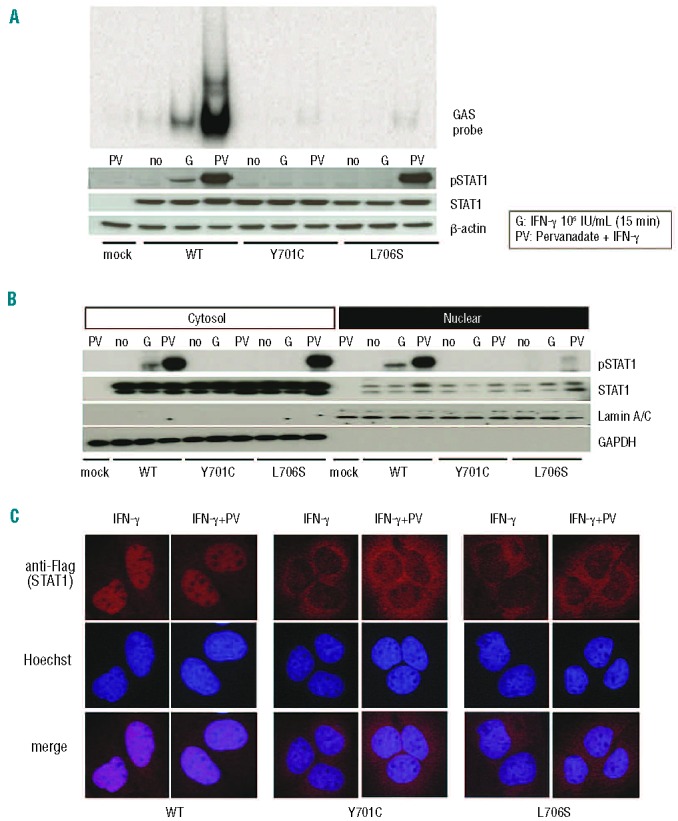
Comparison of the Y701C and L706S mutations. (A) U3C transfectants were stimulated with 105 IU/mL IFN-γ in the presence or absence of pervanadate for 15 min and subjected to immunoblotting and electrophoretic mobility shift assay. The L706S STAT1 protein was phosphorylated to almost normal levels in response to IFN-γ stimulation in the presence of pervanadate, whereas no phosphorylation of the Y701C STAT1 protein was observed. However, L706S STAT1 was still associated with a lack of GAF-DNA binding, even after its phosphorylation had been restored. (B) STAT1 phosphorylation was investigated in the nuclear and cytosolic fractions, by immunoblotting. The phosphorylated L706S STAT1 protein was mostly present in the cytosol fraction. (C) U2OS cells stably expressing Flag-tagged WT, Y701C, or L706S STAT1 were stimulated with IFN-γ for 30 min in the presence or absence of pervanadate and subjected to immunostaining. Nuclear translocation was severely impaired in cells producing either Y701C or L706S STAT1, even after pervanadate treatment. At least two independent experiments were carried out.
