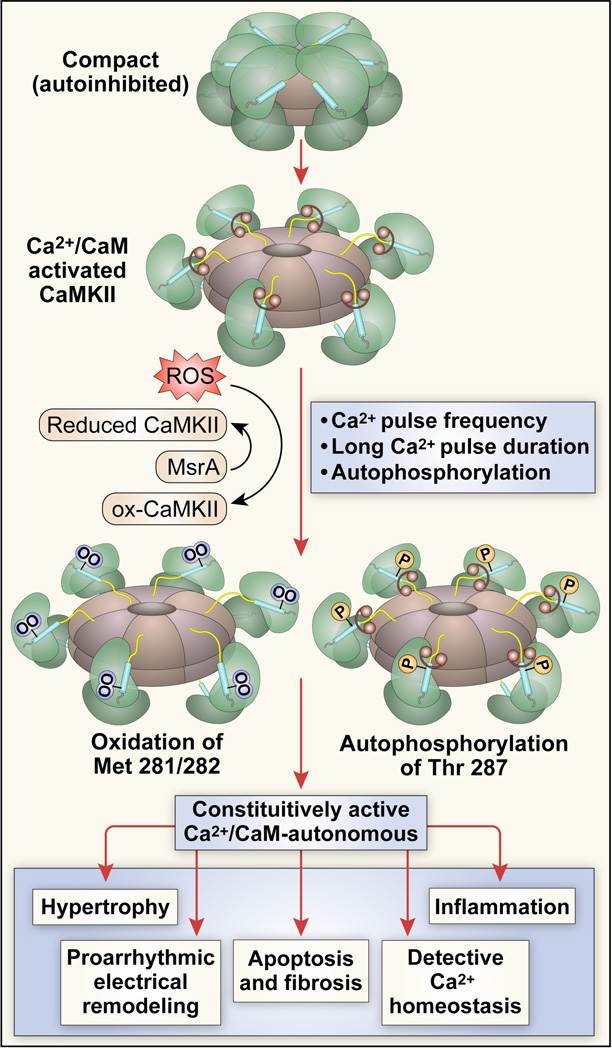
Figure 1. CaMKII becomes constitutively active by autophosphorylation and/or oxidation and constitutively active CaMKII promotes core events important for heart failure and arrhythmias.
The CaMKII holoenzyme consists of two hexameric stacked rings. Under resting conditions the catalytic subunit is conformationally constrained so that CaMKII is inactive (top). CaMKII is initially activated when Ca2+ bound calmodulin (Ca2+/CaM, dumbbell shapes) bind to the regulatory domain (blue segments) causing a more extended conformation where the catalytic domain (green) becomes accessible to substrate proteins and ATP. Sustained increases in Ca2+ lead to autophosphorylation at Thr 287, enhancing the avidity of CaM binding but also inducing a Ca2+/CaM-independent form of CaMKII after CaM unbinding. Oxidation of Met 281/282 induces a Ca2+/CaM-independent form of CaMKII without CaM trapping. Increased levels of autophosphorylated or oxidized CaMKII favor heart failure and arrhythmias, at least in part, by activating hypertrophic and inflammatory gene programs, inducing proarrhythmic electrical remodeling, increasing myocyte death and fibrosis and defective intracellular Ca2+ homeostasis.