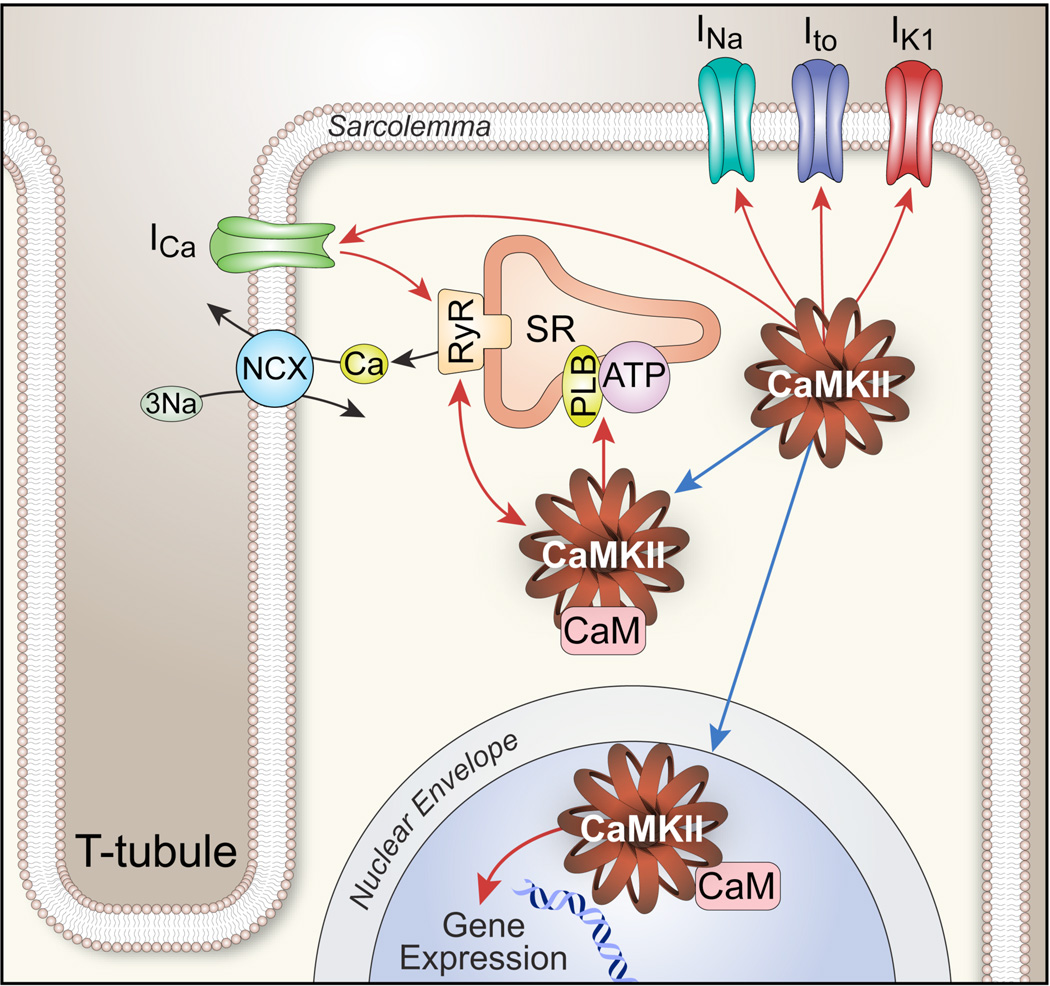
Figure 3. Myocardial cell ultrastructure creates close associations between CaMKII targets important for membrane excitability and intracellular Ca2+ homeostasis.
Activated CaMKII enhances cellular Ca2+ entry by catalyzing phosphorylation of voltage-gated Ca2+ channels (mostly CaV1.2 in ventricle) and ryanodine receptor (RyR2) Ca2+ release channels. CaV1.2 and RyR2 are brought into close association (~10 nm) by CaV1.2 enrichment on T-tubular sarcolemmal invaginations. CaMKII phosphorylation of voltage-gated Na+ channels (mostly NaV1.5 in ventricle) increases subsarcolemmal Na+ concentration that disadvantages the electrochemical driving force for Ca2+ extrusion from the cell by the Na+/Ca2+ exchanger (NCX), leading to increased intracellular Ca2+ concentration.