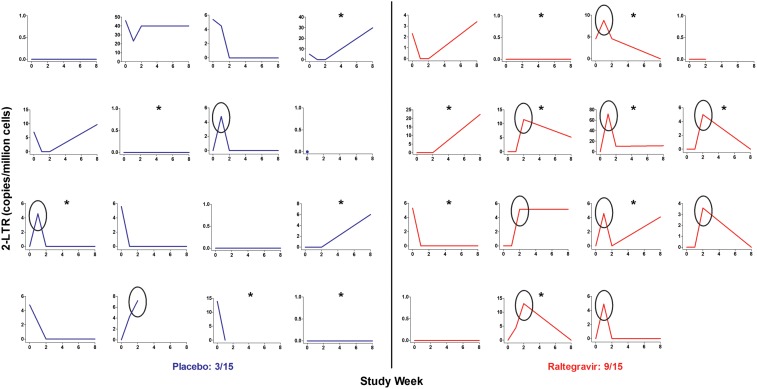
Figure 1.
Increase in the level of 2–long terminal repeat (2-LTR) circles in the raltegravir group. The raltegravir group (red) had a significant increase in the level of 2-LTR circles, compared with the placebo group (blue). The week 1 to week 0 ratio was 8.8-fold higher (P = .0025) and the week 2 to week 0 ratio was 5.7-fold higher (P = .023) in the raltegravir group, compared with the placebo group. Nine of the 15 subjects receiving raltegravir had an increase in the level of 2-LTR circles at weeks 1 or 2, compared with 3 of 15 subjects in the placebo group (P = .060; 1 subject in the placebo group was excluded because of no follow-up 2-LTR circle measurements). Black ovals indicate subjects who had an increase in the level of 2-LTR circles at weeks 1 or 2. Asterisks indicate subjects receiving a protease inhibitor as part of their antiretroviral therapy regimen.