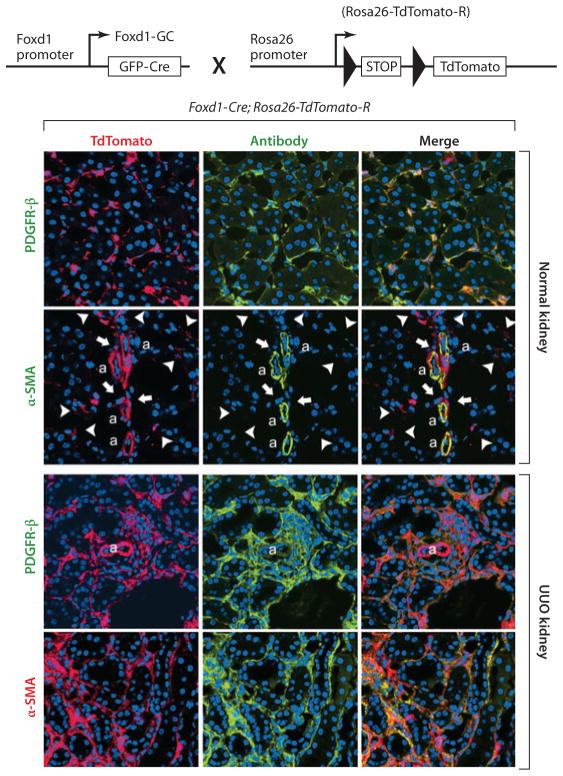
Figure 3.
Genetic fate mapping of mesenchymal progenitors in normal adult and injured kidney by use of the Foxd1-Cre;Rosa26-TdTomato-R mouse. (Top) The cross of the Foxd1–Cre recombinase allele with the TdTomato reporter allele, driven by the universal promoters at the Rosa26 locus. Bigenic mice recombine genomic DNA at the Rosa locus only in cells that have activated Foxd1 in nephrogenesis. (Bottom) Confocal images of kidney cortex in normal adult kidney show large numbers of perivascular cells, all of which coexpress platelet-derived growth factor receptor β (PDGFR-β). Vascular smooth muscle cells of the kidney arterioles are also derived from Foxd1 progenitors and coexpress α–smooth muscle actin (α-SMA) intermediate filament in normal kidney, but none of the Foxd1-derived pericytes (arrowheads) or perivascular fibroblasts (arrows) express α-SMA. In kidney injury [shown here is unilateral ureteral obstruction (UUO) day 7], the pericyte and perivascular fibroblast populations expand and continue to express PDGFR-β. However, the entire expanded population of interstitial Foxd1 progenitor–derived cells coexpress α-SMA, the marker that defines these cells as myofibroblasts. Abbreviations: a, arteriole; GFP, green fluorescent protein.