Figure 1.
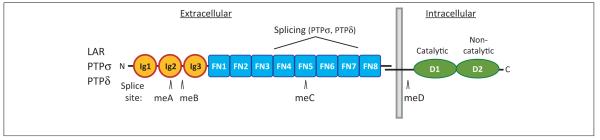
The structure of type IIa receptor-type protein tyrosine phosphatases (RPTPs). Each RPTP contains three extracellular immunoglobulin (Ig)-like domains followed by four or eight fibronectin III (FNIII) domains, depending on alternative splicing, and two intracellular protein tyrosine phosphatase (PTP) domains, the membrane-proximal D1 domain with robust catalytic activity and the membrane-distal D2 domain with residual or no catalytic activity [2–4]. The additional multiple isoforms of RPTPs are generated by alternative splicing of four mini-exons (meA–meD) encoding short amino acid peptides [22,97]. The meA insert with nine or fewer residues is located in the second Ig domain (Ig2), presumably affecting the length of a loop region between the D and E β-strands of Ig2, whereas the meB insert with four residues is located at the end of Ig2 [22,97,98]. The two Drosophila orthologs DLAR and DPTP69D and the single Caenorhabditis elegans ortholog PTP-3 also have two intracellular PTP domains but differ in the number of extracellular Ig and FNIII domains [2,4,31]. The site of constitutive proteolytic processing that generates an extracellular subunit (E-subunit), which remains noncovalently bound to the phosphatase domain subunit (P-subunit) [4], is also indicated.
