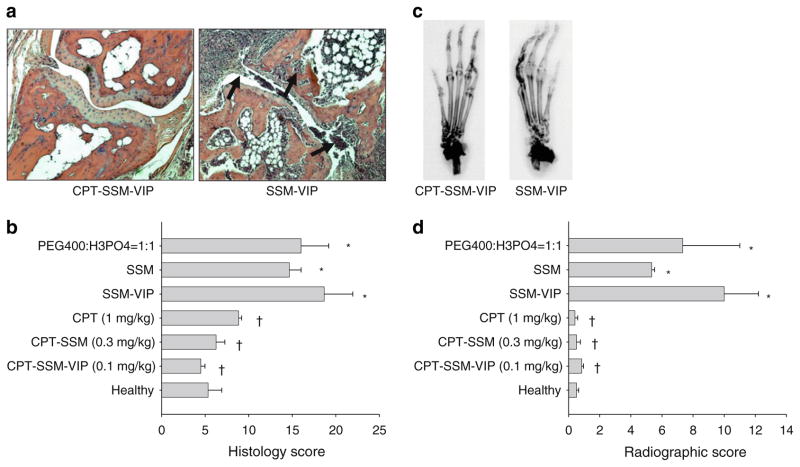
Fig. 3.
CPT-SSM-VIP abrogates CIA-characteristic inflammation of the synovial tissue, cartilage destruction and bone erosion. (a) Representative histopathology joint section of CPT-SSM-VIP-treated (left) and SSM-VIP control (right) mice on Day 60 (magnification ×100). Arrows represent abnormal infiltration of inflammatory cells and bone/cartilage destruction. (b) Average pooled histological scores of paw sections taken from mice injected with various treatments at effective dose levels and controls. (c) Representative radiographs of CPT-SSM-VIP-treated (left) and SSM-VIP control (right) mice on Day 60. (d) Average pooled radiological scores of paw sections taken from mice injected with various treatments at effective dose levels and controls. Results are expressed as mean ± S.E.M. (6 mice/group). *p<0.05 versus normal mice, †p<0.05 versus empty controls.