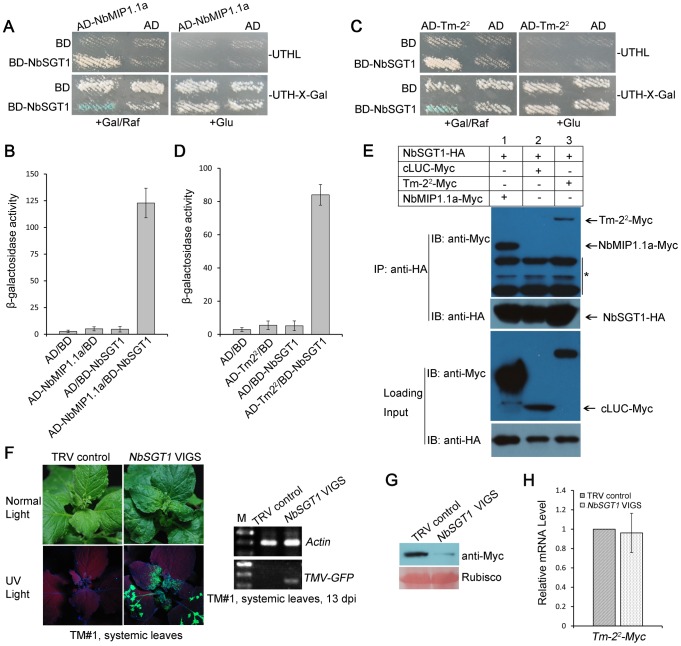
Figure 8. NbSGT1 interacts with NbMIP1.1a and Tm-22 and is required for Tm-22-mediated resistance to TMV.
(A–D) NbSGT1 interacts with both NbMIP1.1a and Tm-22 in yeast. (A, C) Yeast cells harboring AD-NbMIP1.1a (A), AD-Tm-22 (C) transformed with AD-NbSGT1 grew on Leu- selection medium and turned blue on X-gal medium plus Gal/Raf but not on medium plus glucose. Yeast cells transformed with either BD or AD vector alone for control assays showed no growth on Leu− selection medium and remained white on X-gal medium containing either Gal/Raf or glucose. For each experiment, yeast strains were maintained at 28°C for 5 days. (B, D) Quantification of β-galactosidase activities in yeast two-hybrid interactions. (E) NbSGT1 co-immunoprecipitated (co-IP) with NbMIP1.1a and Tm-22. NbSGT1-HA was co-expressed with NbMIP1.1a-Myc or Tm-22-Myc respectively in N. benthamiana leaves through agroinfiltration. Coexpression of NbSGT1-HA and cLUC-Myc was used as a negative control. At 2 dpi, leaf lysates were immunoprecipitated with anti-HA beads, then the immunoprecipitates were assessed by western blotting using anti-Myc (upper panel) and anti-HA antibodies (middle panel). In addition to immunoblotting for co-IP, presence of NbSGT1-HA, NbMIP1.1a-Myc, Tm-22-Myc and cLUC-Myc in the cell lysates were also analyzed (lower panel). * indicates nonspecific bands. (F) Silencing of NbSGT1 in Tm-22 transgenic TM#1 plants caused TMV-GFP spreading into the upper non-inoculated leaves (left). TRV-infected TM#1 plants were used as negative controls. Photos were taken at 10 dpi. RT-PCR was performed to confirm the presence of TMV-GFP in systemic leaves of NbSGT1-silenced TM#1 plants (right). (G) Silencing of NbSGT1 reduced the protein level of Tm-22-Myc. Ponceau Red staining of Rubisco indicates equal loading (lower panel). Experiments were performed three times with three replicated samples in each experiment. (H) Real-time RT-PCR showed that silencing of NbSGT1 had no effect on the expression level of Tm-22-Myc, and Actin mRNA levels were used as the internal control. Data are shown as means ± SD for 3 independent triplicate experiments (Student's t-test).