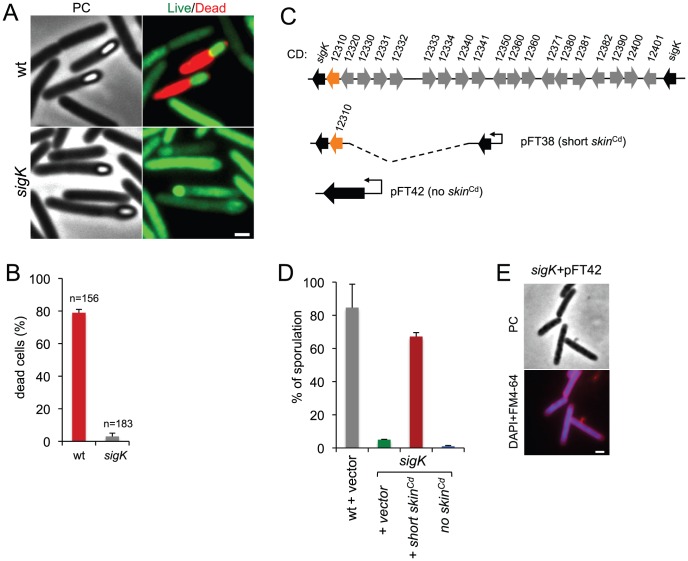
Figure 3. Functional analysis of the sigK gene.
(A) Live/dead assay for the wild type (630Δerm) and the sigK mutant. Shown are phase contrast and the merge between syto 9- (live cells stain; green) and propidium iodide- (dead cells stain; red) stained cells collected at 24 h of growth in SM broth. In the wild type, but not in the sigK mutant, development of spore refractility is accompanied by loss of mother cell viability. (B) Percentage of the sporulating cells of the wild type and sigK mutant strains (i.e., with visible spores) showing signs of mother cell lysis (red; propidium iodide staining) as scored by direct microscopic observation, 24 hours after inoculation in SM medium. Values are the average ± SD of three independent experiments; “n” represents the total number of cells analyzed. (C) The sigK-skin region of the 630Δerm chromosome and plasmids used to complement sigK mutant strain. Replicative plasmid pFT38 carries sigK interrupted by a shorter version of the skin Cd element, which includes the gene (CD12310) for the recombinase (in orange). Replicative plasmid pFT42 carries an uninterrupted sigK gene. The coding sequences are numbered according to the reanotation of the C.difficile genome [91]. (D) Percentage of sporulation for strains 630Δerm (wt), sigK and sigK bearing either pFT38 or pFT42. The indicated percentages are the ratio between the titer of heat resistant spores and the total cell titer, measured 72 h following inoculation in SM medium. Values are the average ± SD of three independent experiments. (E) Fluorescence microscopy showing the phenotype of sigK bearing pFT42. Cells were collected at 72 h of growth in SM broth, stained with DAPI and FM4-64, and viewed by phase contrast (PC) and fluorescence microscopy. Scale bar in (A) and (E), 1 µm.