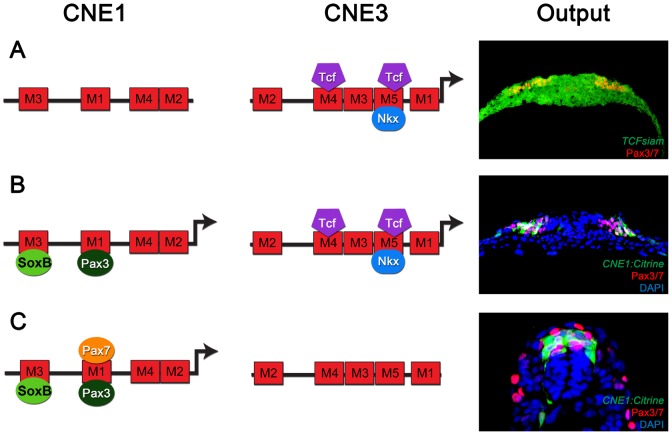
Figure 5. The regulatory logic of Pax3 expression in the neural tube.
(A) Pax3 transcription in the developing CNS is induced by the binding of Wnt pathway effectors, such as Tcf3, to CNE3. Motif4 and Motif5 of CNE3 are likely to mediate this interaction as they contain phylogenetically conserved Tcf/Lef binding sites. The HD binding site within Motif5 is required to repress the activity of this enhancer in vivo. Consistent with this, Nkx6.1 binds to CNE3 in EMSAs and represses endogenous Pax3 expression in vivo. This combination of general activation and medial repression establishes the Pax3 expression domain in the lateral region of the neural plate. (B) Once induced, Pax3 protein binds Motif1 of CNE1 to mediate autoregulation. In addition to autoregulation, CNE1 activity might be restricted to neural tissue by SoxB transcription factors. At early stages of development, both CNE1 and CNE3 are transcriptionally active and may act synergistically to establish the Pax3 expression domain. (C) At later stages, when Pax3 expression reaches its maximum in the neural tube, the majority of dorsal progenitors do not experience active Wnt signaling. Furthermore, the Pax3 expression domain does not share a boundary with ventrally expressed Nkx family members at later stages of development. Our data suggests that a combination of autoregulation and Pax7 mediated positive feedback act to maintain Pax3 expression, seen in vivo as robust CNE1 activity in the absence of CNE3 mediated transcription.