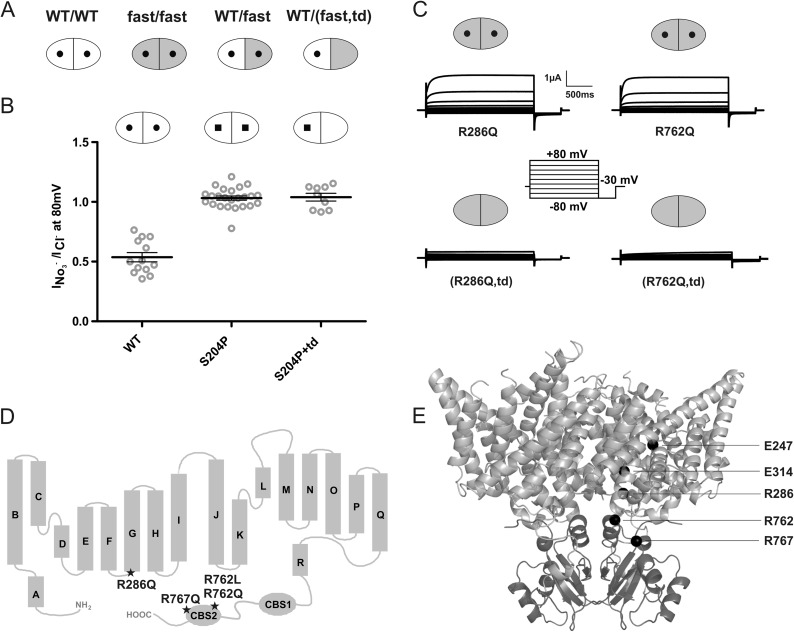
FIGURE 1.
Experimental design and controls. A, schematic presentation of CLC transporter dimers. Each monomer contains an ion permeation pathway. WT subunits are shown in white, subunits with an accelerating point mutation are in gray, and subunits carrying a transport-abolishing point mutation (td; E314A in ClC-7) are symbolized by a lack of the central ”hole.“ From left, WT/WT, fast/fast, WT/fast, and WT/(fast,td) dimers. B, subunits carrying the transport-deficient td mutation do not contribute to currents in WT/td heteromers as probed by differences in nitrate selectivity of WT (round hole) and S204P (square hole) ClC-7 subunits, as indicated by nitrate selectivity of currents that is indistinguishable between S204P/S204P and S204P/td dimers. Thick lines in data point clouds indicate arithmetic mean, and thin lines indicate ± S.E. C, representative voltage clamp traces. The td mutation abolishes currents not only when inserted into WT subunits, but also when inserted into subunits containing the R286Q (lower left trace) or R762Q (lower right trace) mutations. D, topology model of ClC-7 showing the localization of the fast mutations ClC-7R286Q, ClC-7R762Q, ClC-7R762L, and ClC-7R767Q. All these mutations underlie human osteopetrosis. E, mutations mapped into the crystal structure of algal CmClC. The transmembrane domain is shown in light gray, and the CBS domains are in dark gray. Positions of the gating (Glu-247 in ClC-7, Glu-210 in CmClC) and proton (Glu-314 in ClC-7, Thr-269 in CmClC) glutamates and of residues Arg-286, Arg-762, and Arg-767 (in ClC-7, corresponding to Leu-241, Val-680, and Ser-685, respectively, in CmClC) are shown as black spheres, for clarity only in one of the two subunits.