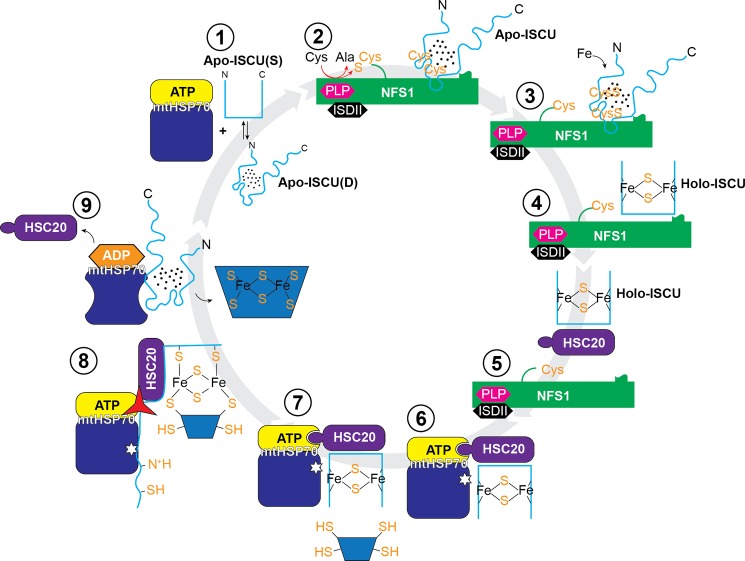
FIGURE 12.
Working model for human mitochondrial Fe-S cluster biogenesis. 1, apoISCU in D⇄S equilibrium. 2, complex formed between the cysteine desulfurase complex (NFS1-ISD11) and the D-state of ISCU. 3, sulfur delivered to Cys residues of ISCU. 4, addition of iron to form a [2Fe-2S] cluster stabilizes the S-state of ISCU. 5, the co-chaperone (HSC20) binds to holo-ISCU displacing the cysteine desulfurase complex. 6, the J-domain of HSC20 binds to the ATPase domain of the chaperone (mtHSP70), bringing holo-ISCU close to the chaperone. 7, an acceptor protein containing free Cys -SH groups approaches. 8, attack of cysteine residues from the acceptor protein liberates residues of ISCU that bind to the chaperone leading to activation of its ATPase activity. 9, conversion of ATP to ADP leads to a conformational change in the substrate binding domain of the chaperone, which then binds preferentially to the D-state of ISCU releasing the holo-acceptor protein and HSC20. 1, exchange of mtHSP70-bound ADP with ATP (which involves an exchange factor, not shown) leads to the release of ISCU, which resumes its equilibrium between the S- and D-states.