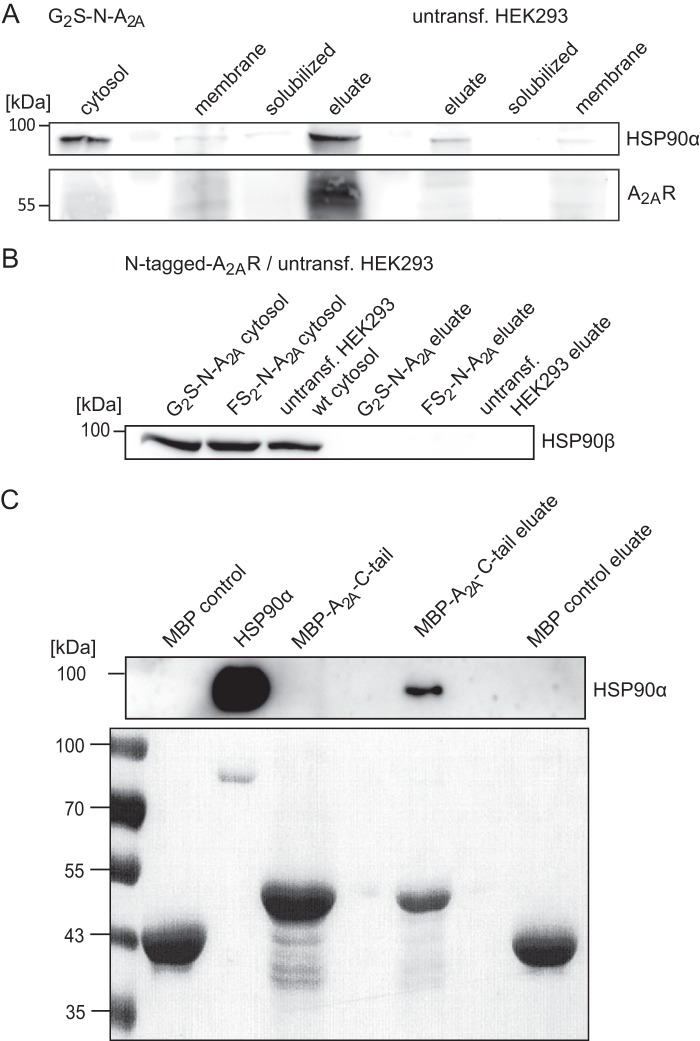
FIGURE 4.
Co-affinity precipitation of N-terminally tagged A2A receptor and HSP90. Membranes and a cytosolic fraction were prepared from cells stably expressing the tagged version of the A2A receptor G2S-N-A2AR and from untransfected HEK293 cells. Membranes were solubilized with DDM, and the soluble extract was mixed with streptavidin beads. After three washes, bound proteins were released by heat denaturation in reducing sample buffer. Volume-corrected aliquots (i.e. corresponding to 2.5 × 105 cells) of the cytosolic fraction, the membrane fraction, the solubilized material, and the final eluate were resolved by denaturing electrophoresis and immunoblotted for HSP90α (A, top), HSP90β (B), and the A2A receptor (bottom; visualized with the anti-A2A receptor). C, maltose-binding protein (MBP control) or MBP-A2A-C-tail fusion protein was incubated with recombinant HSP90α and bound to amylose resin. After two washes, bound proteins were released by heat denaturation in reducing sample buffer. Volume-corrected aliquots (50% of the original reaction) were immunoblotted for HSP90α (C, top). The proteins were also visualized by Ponceau staining of the nitrocellulose membrane to document the amount of MBP employed as input and recovered in the eluate (C, bottom).