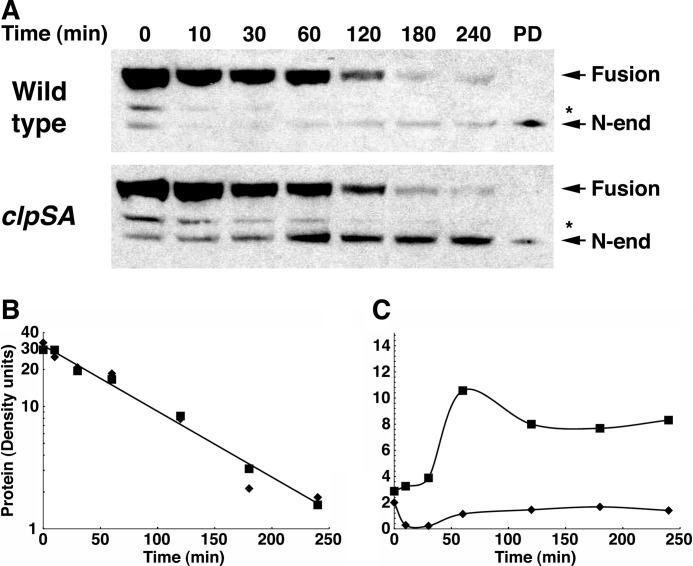
FIGURE 5.
Formation and degradation of the N-end rule fragment of MreB. Wild-type and ΔclpSA cells expressing the MreB-AZ fusion were grown to an A600 of 0.8, and chloramphenicol was added to prevent further synthesis. At the times indicated, aliquots of the culture were treated with TCA to stop metabolic and enzymatic activities and to precipitate the protein. After separation by SDS-PAGE, MreB-AZ was detected by Western blotting with HRP-conjugated anti-rabbit IgG, which binds to the AZ domain. A, turnover of MreB-AZ. The full-length fusion protein is indicated as well as the shorter N-end rule substrate that is formed by cleavage at the pro-N-degrons in MreB. The identity of the latter was confirmed in a separate experiment by N-terminal sequencing of the protein isolated on a ClpS affinity column, which was loaded as a reference in the lane marked PD. The cleavage site in the fragment indicated by an asterisk is not known. B, half-life of MreB-AZ in wild-type and clpSA mutant cells. The protein bands detected by Western blotting were scanned, and the protein remaining at each time point was determined from the density. Integration and calculation were performed using the program NIH ImageJ. The full-length protein was cleaved with a half-life of about 1 h in both wild-type (diamonds) and clpSA cells (squares). C, quantitation of the cleaved MreB-AZ protein. The amount of cleaved MreB-AZ at each time was measured by densitometry as above. The N-degron-containing fusion accumulated in the clpSA mutant (25–30% of the original fusion) but not in the wild-type cells. The slight stabilization of the N-end rule form at later times is due to the loss of ClpA, which is unstable and lost from the cells during the chloramphenicol chase.