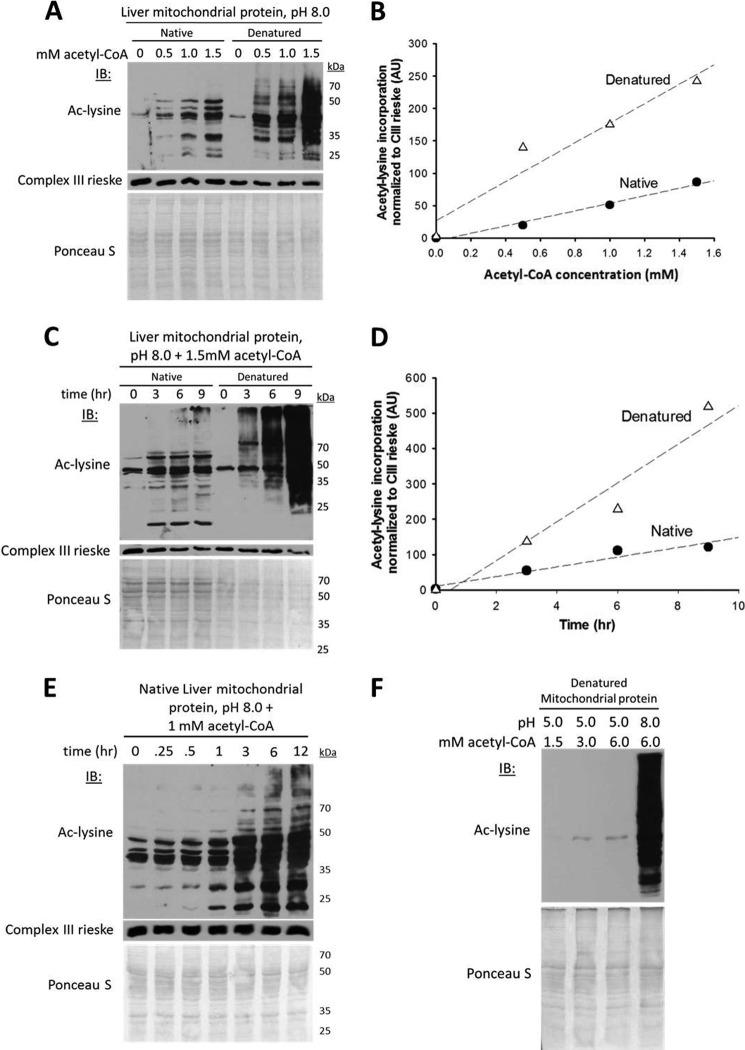
FIGURE 2.
Nonenzymatic Nϵ-acetylation in the chemical conditions of the mitochondrial matrix. A, acetyl-CoA concentration-dependent increases in mitochondrial protein acetylation under native and denatured conditions. The mouse liver mitochondrial extracts (30 μg) were incubated over a range of acetyl-CoA concentrations previously determined to occur in mitochondria (15, 16) (0.1–1.5 mm, and 1 μl of matrix volume/mg of mitochondrial protein) for 6 h at 37 °C with gentle mixing. For denatured samples, mitochondrial extracts (30 μg) were heated at 95 °C for 10 min prior to the addition of acetyl-CoA. IB, immunoblot; ac-lysine, acetyl-lysine. B, quantification of densitometry data in A normalized to Complex III. The control reaction with the smaller arbitrary value was set to zero, and the remaining data points were transformed accordingly. AU, arbitrary units. C, time-dependent incorporation of acetyl groups into native and denatured mitochondrial protein lysine residues. D, quantification of the densitometry data in C normalized to Complex III. E, time-dependent incorporation of acetyl groups into native mitochondrial protein over a broader range of time points. F, acetylation is not caused by the denaturation process and is pH-dependent. In A, C, E, and F, the nitrocellulose membrane used for immunoblotting was stained with the nonspecific protein marker Ponceau S to show equal loading as indicated.