FIGURE 5.
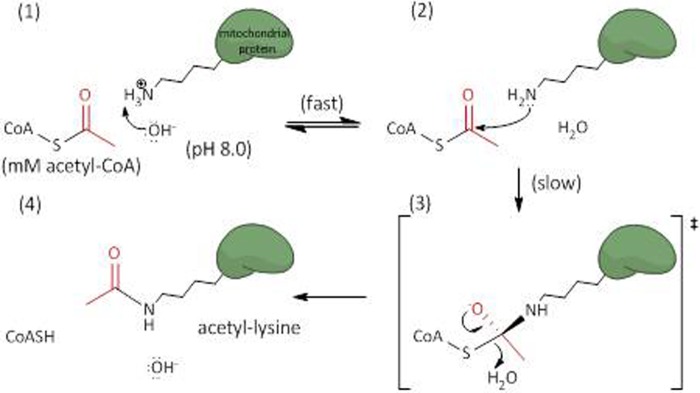
Schematic illustrating the specific base catalyzed acyl transfer mechanism. The alkaline environment of the mitochondrial matrix reflects an increased concentration of hydroxide ions (OH−) free to abstract protons from accessible lysine residues (panel 1). The deprotonated lysine can then perform a nucleophilic attack on the terminal carbonyl carbon of the acyl-CoA (panel 2). A putative tetrahedral intermediate is formed (panel 3), which then collapses, displacing the thioester bond and leaving an acylated (acetylated) lysine, CoASH, and hydroxide (panel 4). This mechanism was adapted from that of the GCN5 acetyltransferase, which employs a general base catalyst to deprotonate lysine residues and initiate acetyl transfer (19).
