FIGURE 1.
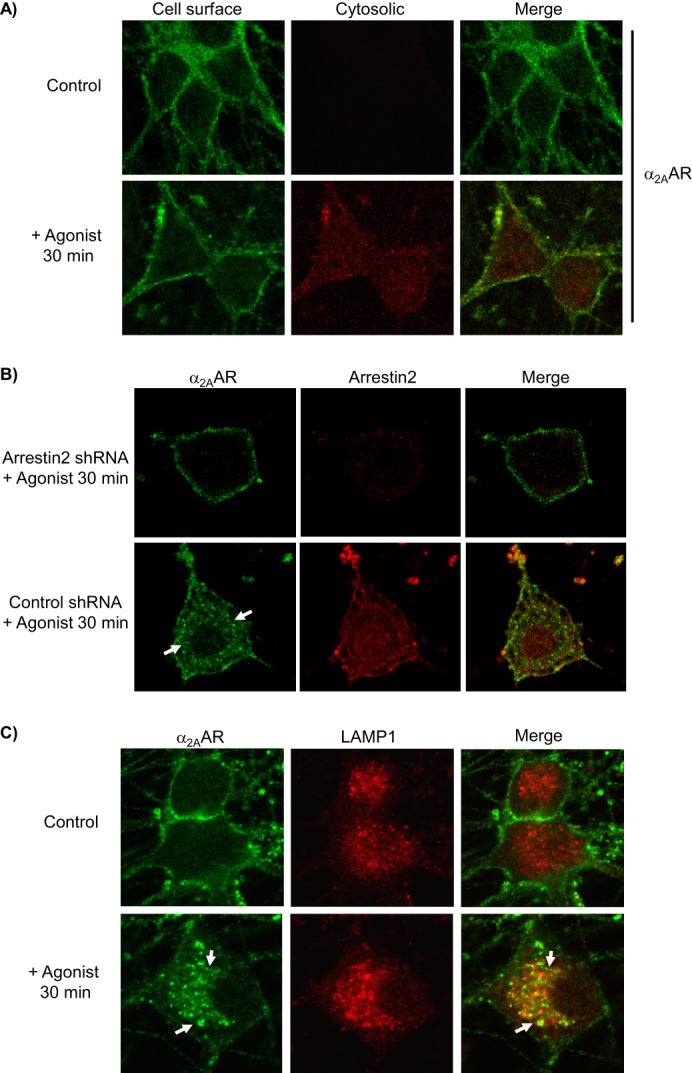
Evaluation of arrestin-dependent agonist-stimulated α2AAR endocytosis in native neurons. All immunostaining experiments detect endogenously expressed HA-α2AARs. A, neurons were subjected to primary antibody prelabeling of surface α2AARs prior to stimulation, followed by saturation of labeled surface receptors with AlexaFluor488-conjugated secondary labeling, and then permeabilization and cytosolic AlexaFluor594-conjugated secondary labeling. Cytosolic labeling was seen only in stimulated cells, indicating endocytosis of prelabeled surface α2AARs. B, endocytosis was assayed in arrestin3-null neurons, transduced with either arrestin2 shRNA (to achieve complete ablation of arrestins) or control shRNA. Analysis revealed that arrestin2 shRNA resulted in a 69 ± 5.8% reduction in arrestin2 immunoreactivity. α2AARs were detected by primary antibody prelabeling followed by permeabilization and AlexaFluor488-conjugated secondary labeling. In control but not arrestin2 shRNA cells, agonist stimulation resulted in significant α2AAR endocytosis, indicated by the appearance of intracellular punctae containing internalized receptors (arrows), as well as a partial co-localization of α2AARs with arrestin2. C, endocytosis was additionally observed by monitoring α2AAR co-localization with the late endolysosomal pathway marker LAMP1; prelabeling of α2AARs was done as in A, with the addition of co-immunostaining for LAMP1. In agonist-stimulated but not unstimulated control neurons, endocytosis was seen as indicated by the appearance of intracellular punctae which exhibited co-localization with LAMP1 (arrows). Confocal images are representative of at least three independent samples.
