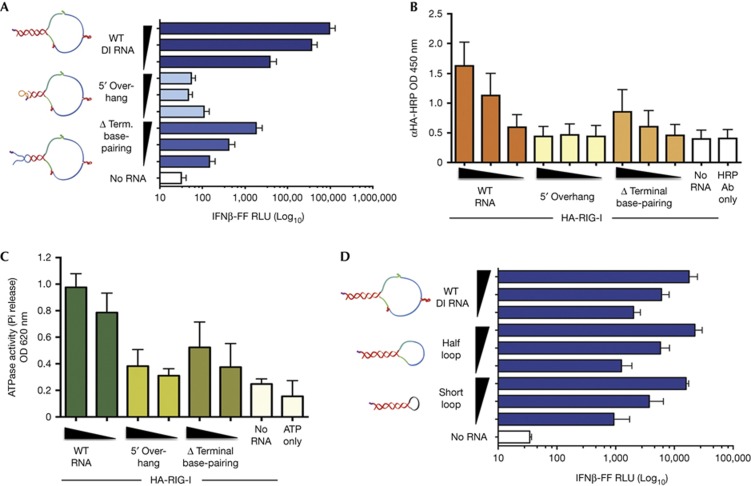
Figure 2.
Exposed 5′-ppp and terminal dsRNA, but not loop structure, are important features of SeV DI RNA for RIG-I-mediated IFN-I activation. (A) 25 fmol and five-fold dilutions of WT, 5′ overhang and Δ terminal base-pairing RNAs were transfected or not into 293T-IFNβ-FF-Luc cells and 24 h later IFNβ promoter-driven luciferase activity was measured by a luciferase assay. (B) 500 fmol and three-fold dilutions of Biotin-UTP-labelled WT, 5′ overhang and Δ terminal base-pairing RNAs were immobilized onto NeutrAvidin-coated wells and incubated with lysates from HA-RIG-I-expressing 293T cells. The levels of bound RIG-I were determined by measuring the absorbance/HRP activity of HA-HRP antibody. (C) 0.5 μg of purified His-HA-RIG-I was incubated with 100 or 50 fmol of WT, 5′ overhang, Δ terminal base-pairing or no RNA in the presence of 0.5 mM ATP and 2.5 mM Mg2+ at 37 °C for 25 min. Released phosphates were measured using Malachite Green-based reagent at an absorbance of 620 nm. (D) 25 fmol and five-fold dilutions of WT, half-loop and short loop RNAs were transfected or not into 293T-IFNβ-FF-Luc cells and 24 h later IFNβ promoter-driven luciferase activity was measured by a luciferase assay. Data are representative of at least three independent experiments and error bars indicate mean±s.d. Log10 in Figs 2A and 2D refers to the scale on the x-axis. 5′-ppp, 5′-triphosphate; DI, defective interfering; dsRNA, double-stranded RNA; HRP, horseradish peroxidase; IFNβ, interferon β; SeV, Sendai virus; WT, wild type.