Abstract
Background:
The Great Britain (GB) Asbestos Survey is a prospective cohort of asbestos workers in GB. The objective of this study was to investigate determinants of mesothelioma latency, paying particular attention to indicators of intensity of asbestos exposure such as occupation, sex, and presence of asbestosis.
Methods:
The analysis included members of the cohort who died with mesothelioma between 1978 and 2005. The primary outcome was the latency period defined as the time from first occupational exposure to asbestos to death with mesothelioma. Generalised gamma accelerated failure-time models were used to estimate time ratios (TRs).
Results:
After excluding missing data, there were 614 workers who died with mesothelioma between 1978 and 2005. Total follow-up time was 9280 person-years, with a median latency of 22.8 years (95% confidence interval (CI) 16.0–27.2 years). In the fully adjusted model, latency was around 29% longer for females compared with males (TR=1.29, 95% CI=1.18–1.42), and 5% shorter for those who died with asbestosis compared with those who did not (TR=0.95, 95% CI=0.91–0.99). There was no evidence of an association between latency and occupation.
Conclusion:
This study did not find sufficient evidence that greater intensity asbestos exposures would lead to shorter mesothelioma latencies.
Keywords: mesothelioma latency, occupational exposure, asbestos, Great Britain, intensity hypothesis
Mesothelioma is a relatively rare form of cancer that is almost exclusively caused by exposure to asbestos. The two main forms of mesothelioma are pleural mesothelioma, which affects the covering of the lungs and accounts for around 75% of cases, and peritoneal mesothelioma, which affects the abdomen (Cancer Research UK, 2012). Although rare, mesothelioma has extremely poor prognosis and Great Britain (GB) has one of the highest incidence rates in the world (Bianchi and Bianchi, 2007). In 2010, there were 1946 mesothelioma deaths among men in GB and 401 among women (Health and Safety Executive, 2013b).
Despite controls on the use of asbestos dating as far back as the 1960s, and the final national ban on the importation and use of asbestos in 1999, the annual number of deaths from mesothelioma in GB has continued to rise (Health and Safety Executive, 2013b). This can be attributed to the long latency period associated with mesothelioma, which has been estimated to have a median duration of around 32 years (Lanphear and Buncher, 1992). So there will be a substantial delay before the potential benefits of any controls on asbestos use will start to emerge. It has been estimated that the number of mesothelioma cases among males in GB is expected to peak at around 2100 cases (as an upper limit) in 2016 before starting to reduce (Tan and Warren, 2011).
The latency period of mesothelioma is long but it is also highly variable, and can range anywhere from 13 to 70 years (Lanphear and Buncher, 1992). A greater understanding of the factors that determine the duration between exposure to asbestos and development of mesothelioma could help to improve predictions of the future number of cases; it could help to attribute new cases to past exposures (for example, to a particular time period or occupation), and it could aid the understanding of the disease process. However, very few studies have investigated this variability in detail. Differences in mesothelioma latency that have been observed—for example, differences by occupation (Bianchi et al, 1997), sex (Haber and Haber, 2011), and source of exposure (Marinaccio et al, 2007)—are often attributed to differences in the intensity of exposure to asbestos. This ‘intensity hypothesis' proposes that there is an inverse relationship between the intensity of asbestos exposure and the length of the latency period. However, the data supporting this are sparse and often conflicting, and some of the observed differences could be due to the definition of latency itself. Latency is associated with age at exposure, follow-up time, and mortality rate, but these associations are purely a consequence of the definition of latency (the time between first exposure to incidence/death) rather than a true effect (Peto, 1985). For example, those more recently exposed to asbestos would not generally have experienced sufficient follow-up time to develop mesothelioma, and so only those with shorter latencies would have developed the disease within the study time frame; this would make the latency period appear to be shorter in more recent time periods.
The objective of this study was to investigate the determinants of mesothelioma latency among a cohort of asbestos workers, paying particular attention to indicators of intensity of asbestos exposure such as occupation, sex and presence of asbestosis (diffuse pleural fibrosis caused by asbestos). Asbestos insulation workers (Williams et al, 2007) and those who develop asbestosis (Lemen and Dement, 1980; Jamrozik et al, 2011) tend to have experienced greater exposure to asbestos than others, and women are often thought to have had historically lower exposures than men. Therefore, if the hypothesis of an inverse relationship between the intensity of asbestos exposure and the length of the latency period is true, then it is expected that those employed in the insulation sector, males, and those with asbestosis would have shorter latencies than other asbestos workers.
Materials and methods
The GB Asbestos Survey is a prospective cohort established in 1971 to monitor the long-term health of asbestos workers attending regular medical examinations in GB. Recruitment started in 1971 and continues to this day. The study is described in detail elsewhere (Harding and Wegerdt, 2007; Harding et al, 2009; Harding and Frost, 2010), and so is briefly summarised here.
Asbestos workers attending medical examinations under the asbestos regulations in place in GB at the time were eligible to participate. The asbestos regulations changed over time and, as a consequence, so too did the inclusion criteria for the GB Asbestos Survey. Under the 1969 Asbestos Regulations, all employees at workplaces covered by the regulations were invited to attend voluntary medical examinations, which were undertaken every 2 years while the individual was in employment. The 1983 Asbestos Licensing Regulations made the examinations statutory, and expanded the inclusion criteria to all individuals working with listed materials. Finally, in 1987, the Control of Asbestos at Work Regulations extended the requirement for statutory medical examinations to all workers occupationally exposed to asbestos above a certain action level. There have been other asbestos regulations since 1987 (Lowe et al, 2004; Oracle Solutions, 2013), but none would substantially impact upon those requiring medical examinations. From 1971 when attending the medical examination, the workers were invited to participate in the GB Asbestos Survey and asked to complete a short questionnaire.
The study questionnaire was completed at each medical examination attended by the participant. This collected basic information on first occupational exposure to asbestos, type of job currently held, and current smoking status. No information on actual asbestos exposure was collected. Health outcomes were based on mortality and cancer incidence, and were ascertained by flagging the study participants with the Health and Social Care Information Centre (HSCIC) for England and Wales, and the General Register Office for Scotland (GROS). The GB Asbestos Survey was also linked to the GB Mesothelioma Register and the GB Asbestosis Register, both of which are maintained by the Health and Safety Executive (HSE) (2013a). Data from the GB Asbestosis Register were only available from 1978 onwards since they were not held in electronic format before this (Harding and Darnton, 2010).
The British Medical Association Research Ethics Committee gave approval for the GB Asbestos Survey, and the Office for National Statistics Caldicott Guardian approved the linkage with the GB Mesothelioma Register and the GB Asbestosis Register. The London School of Hygiene and Tropical Medicine Research Ethics Committee approved this study.
Statistical methods
For the purpose of this analysis, latency is defined as the time from first occupational exposure to asbestos to death with mesothelioma. The date of first occupational exposure to asbestos was taken to be the date of the first examination or the date of first exposure as recorded on the study questionnaire, whichever occurred first. The GB Mesothelioma Register was used to identify those who died with mesothelioma and the death certificate ascertained the date of death.
Variables of interest were sex, smoking status, occupation, year of first exposure, age at first exposure, duration of exposure, type of mesothelioma, and death with asbestosis. Occupation, sex, and death with asbestosis were considered indicators of intensity of asbestos exposure. Smoking status was categorised as never, former, or current. The smoking status of the participant could change during follow-up if they had completed more than one questionnaire, and so the analysis used the smoking status recorded most often. Occupation was categorised into the broad occupational groups of manufacturing, removal, insulation, and ‘other' work, and the occupation reported most often was used. Year of first exposure (10-year categories from <1940 to 1980+) and age at first exposure (10-year categories from <20 to 50+ years of age) were derived from the date of first exposure as defined above. Duration of exposure was calculated from the date of first exposure to the date of last exposure. Medical examinations were required every 2 years, and so the date of last occupational exposure was assumed to be 2 years after the participant's final examination. Duration of exposure was entered as a time-dependent covariate (10-year categories from <10 years to 40+ years). Participants who died with asbestosis were identified using the GB Asbestosis Register. Type of mesothelioma was categorised into pleural (ICD-8 163.0; ICD-9 163.0–163.9; ICD-10 C45.0), peritoneal (ICD-8/9 158.0–158.9; ICD-10 C45.1), pleural plus peritoneal, or ‘unspecified'. Mesothelioma type was missing in the GB Mesothelioma Register for the majority of cases among the cohort (83% missing). Therefore, death certificates (underlying and associated causes) and cancer registrations were used to supplement this information.
Mesothelioma latency was analysed as survival (or time to event) data—that is, time from first occupational exposure to asbestos to death with mesothelioma. Individuals became ‘at risk' at the date of first occupational exposure to asbestos, but did not come under observation and start contributing to the analysis until entry into the study or 1978, whichever came later. Those who died with mesothelioma between 1978 and the end of 2005, and who had complete information on all of the variables of interest, were included in the analysis. Note that for the purpose of this particular analysis, follow-up started in 1978 rather than when the GB Asbestos Survey started in 1971, due to the lack of availability of the GB Asbestosis Register before this date. All analyses were undertaken using Stata/SE 12.1 for Windows (StataCorp, 2012).
Characteristics of the full cohort, of those who died with mesothelioma, and of those who died from other causes, were examined and are presented in terms of their frequency distributions or their median plus interquartile range. The latency period was summarised using Kaplan–Meier survival curves and the smoothed hazard estimate.
Accelerated failure-time (AFT) models were used to investigate the association between mesothelioma latency and the variables of interest. Accelerated failure-time models are an alternative parameterisation of parametric survival models, which put the emphasis on the time to an event (Cleves et al, 2004). The exponentiated coefficients provide estimates of ‘time ratios' rather than hazard ratios, which is a more intuitive measure when analysing time to an event. A time ratio (TR) shows the proportional change in survival time (in this case latency) for each unit increase in the variable; a TR less than one corresponds to a decrease in survival, and a TR greater than one corresponds to an increase in survival.
The exponential, Weibull, log-normal, log-logistic, and generalised gamma distributions can all be chosen as the survival distribution for an AFT model (Cleves et al, 2004). To choose the most appropriate distribution, an empty AFT model was fitted to the data using each distribution in turn, and then the goodness-of-fit of the different models were compared using the Akaike Information Criterion (AIC). In addition, the exponential, Weibull and log-normal distributions are all nested within the generalised gamma distribution, and so the model fit was also compared using the likelihood-ratio test (LR test) (Cleves et al, 2004). Both the AIC values and the LR tests provided strong evidence that the generalised gamma AFT model provided the best fit to the data, and so this survival distribution was used throughout.
The variables of interest were entered into separate generalised gamma AFT models as categorical variables, and their association with mesothelioma latency was tested using the LR test. Time ratios were additionally estimated with adjustment for year at first occupational exposure to asbestos and age at first exposure, to adjust for the known relationship between latency and duration of follow-up and age at exposure. A ‘full' AFT model, which included all variables of interest, is also presented. Adjusted median latencies were estimated using predicted values from the full AFT model. The overall fit of the full AFT model was assessed using Cox–Snell residuals.
Additional industrial breakdown was available within manufacturing and ‘other' occupations. Results are therefore also shown for the full AFT model using the more detailed industrial breakdown rather than main occupation. The additional breakdown resulted in some groups containing a small number of cases, which could result in unreliable TRs. Hence, TRs are not presented when the number of mesothelioma deaths was fewer than five.
Two main sources of potential bias were identified a priori. The first related to excluding follow-up from 1971 to 1977, as the linkage to the GB Asbestosis Register could only be performed from 1978 onwards. Therefore, the analyses were repeated using the full data to ensure that the restriction did not introduce bias. Second, cases of mesothelioma with a latency period of less than 10 years were excluded and the analysis repeated.
Results
From 1971 to 2005, the GB Asbestos Survey recruited 98 912 asbestos workers, 647 of whom had died with mesothelioma. The number of cases of mesothelioma differed from that previously published (Harding and Darnton, 2010), as two cases had subsequently been removed due to an error in cross-matching the GB Asbestos Survey and the GB Mesothelioma Register.
Restricting follow-up to 1978 onwards resulted in 98 447 asbestos workers and 632 cases of mesothelioma. Of these, 3487 (3.5%) workers in total and 18 (2.8%) of those who had died with mesothelioma were missing main smoking status and/or main occupation. This was a small percentage and so excluding them from the analysis was unlikely to bias results. This left 614 workers who died with mesothelioma between 1978 and 2005 for analysis, who were followed-up for a total of 9280 person-years.
Table 1 shows the characteristics of the full cohort, of those who died with mesothelioma, and of those who died from other causes. Those who died with mesothelioma had a lower proportion of current smokers than those who died from other causes (56% and 68%, respectively), had a greater proportion of removal workers (33% and 19%, respectively) and insulation workers (21% and 10%, respectively), tended to have had an earlier year of first exposure (median 1958 and 1966, respectively), been younger at first occupational exposure to asbestos (median 23 and 35 years, respectively), and had a longer duration of exposure (median 26.5 and 15.9 years, respectively). Most differences can be attributed to the long latency period associated with mesothelioma. In addition, those who died with mesothelioma had a greater proportion of deaths with asbestosis than those who died from other causes (14% and 3%, respectively). More cases of mesothelioma were classified as pleural mesothelioma than peritoneal mesothelioma (49% and 22%, respectively), and just over a quarter (28%) could not be classified.
Table 1. Characteristics of the full cohort of British asbestos workers, and of those who died with mesothelioma (1978–2005).
| Characteristic | Full cohort | Died with mesothelioma | Died from other causes |
|---|---|---|---|
| Total number |
94 960 |
614 |
14 009 |
| Total follow-up time (years) |
1 636 004 |
9280 |
209 663 |
|
Sex,
n
(%) |
|
|
|
| Male | 90 640 (95) | 596 (97) | 13 202 (94) |
| Female |
4320 (5) |
18 (3) |
807 (6) |
| Age at entry, median (IQR) |
33 (25–44) |
47 (40–53) |
50 (42–57) |
| Year of entry, median (IQR) |
1985 (1977–1992) |
1976 (1975–1982) |
1976 (1974–1979) |
|
Main smoking status during follow-up,
n
(%) |
|
|
|
| Current | 54 744 (58) | 344 (56) | 9585 (68) |
| Former | 16 217 (17) | 168 (27) | 2908 (21) |
| Never |
23 999 (25) |
102 (17) |
1516 (11) |
|
Main occupation during follow-up,
n
(%) |
|
|
|
| Manufacturing | 27 034 (28) | 203 (33) | 7491 (53) |
| Removal | 50 545 (53) | 200 (33) | 2662 (19) |
| Other | 12 383 (13) | 82 (13) | 2446 (17) |
| Insulation |
4998 (5) |
129 (21) |
1410 (10) |
| Year of first occupational exposure to asbestos, median (IQR) |
1981 (1970–1991) |
1958 (1951–1967) |
1966 (1954–1974) |
| Age (years) at first occupational exposure to asbestos, median (IQR) |
27 (21–36) |
23 (16–34) |
35 (24–45) |
| Duration (years) of occupational exposure to asbestos, median (IQR) |
6.0 (2.0–16.1) |
26.5 (16.6–35.4) |
15.9 (6.7–28.5) |
|
Died with asbestosis,
n
(%)a |
|
|
|
| No | 14 159 (97) | 526 (86) | 13 630 (97) |
| Yes |
467 (3) |
88 (14) |
379 (3) |
|
Mesothelioma type,
n
(%) |
|
|
|
| Pleural | NA | 301 (49) | NA |
| Peritoneal | NA | 132 (22) | NA |
| Pleural+peritoneal | NA | 10 (2) | NA |
| Not specified | NA | 171 (28) | NA |
Abbreviations: IQR=interquartile range; NA=not applicable.
Total deaths in full cohort=14 623.
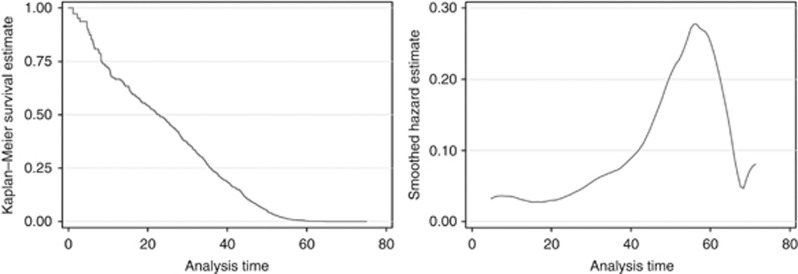
Figure 1 shows the survival and hazard curves for the asbestos workers who died with mesothelioma. Median latency was 22.8 years (95% confidence interval (CI) 16.0–27.2 years), and the probability of latency being less than 10 years was 0.2795 (95% CI=0.1945–0.3908). The hazard peaked at around 55 years after first exposure to asbestos (Figure 1).
Figure 1.
Kaplan–Meier estimate (left) and the smoothed hazard function (right) for British asbestos workers who died with mesothelioma (1978–2005).
Table 2 shows the median latency and crude TRs for the variables of interest. Year of first exposure, age at first exposure, and duration of exposure were all highly statistically significantly associated with latency (all LR-test P<0.001). Median latency tended to decrease with increasing year of first exposure and age at first exposure, and increase with duration of exposure (Table 2). There was strong evidence of an association between latency and sex, with females tending to have a latency that was 26% longer than that of males (TR=1.26, 95% CI=1.11–1.44; Table 2). There was no evidence that main smoking status, main occupation, mesothelioma type, or whether the worker died with asbestosis was associated with mesothelioma latency (all LR-test P>0.10).
Table 2. Median mesothelioma latency and crude time ratios for mesothelioma latency among British asbestos workers (1978–2005).
| Characteristic | No. of deaths | Person- years at risk | Median latency (years) | (95% CI) | Crude time ratio | (95% CI) | LR test |
|---|---|---|---|---|---|---|---|
| Total |
614 |
9280 |
22.8 |
(16.0–27.2) |
NA |
NA |
NA |
|
Sex |
|
|
|
|
|
|
P<0.001 |
| Male | 596 | 8995 | 22.2 | (15.5–27.0) | 1.00 | (ref) | |
| Female |
18 |
285 |
28.2 |
(14.0–34.1) |
1.26 |
(1.11–1.44) |
|
|
Main smoking status |
|
|
|
|
|
|
P=0.784 |
| Current | 344 | 5309 | 24.8 | (11.8–28.2) | 1.00 | (ref) | |
| Former | 168 | 2440 | 21.5 | (8.3–31.2) | 1.00 | (0.95–1.06) | |
| Never |
102 |
1531 |
19.4 |
(7.6–28.1) |
0.98 |
(0.91–1.05) |
|
|
Main occupation |
|
|
|
|
|
|
P=0.982 |
| Manufacturing | 203 | 3162 | 24.8 | (8.2–31.2) | 1.00 | (0.94–1.08) | |
| Removal | 200 | 2899 | 19.6 | (13.9–26.8) | 0.99 | (0.93–1.06) | |
| Other | 82 | 1305 | 25.5 | (6.2–30.2) | 1.01 | (0.92–1.10) | |
| Insulation |
129 |
1915 |
20.3 |
(2.2–31.3) |
1.00 |
(ref) |
|
|
Year of first exposure |
|
|
|
|
|
|
P<0.001 |
| <1940 | 40 | 303 | 48.1 | (44.6–50.1) | 1.00 | (ref) | |
| 1940−1949 | 96 | 1325 | 47.7 | (45.9–50.0) | 0.94 | (0.87–1.00) | |
| 1950−1959 | 216 | 3347 | 40.6 | (38.3–42.0) | 0.83 | (0.78–0.88) | |
| 1960−1969 | 145 | 2521 | 33.0 | (31.3–34.4) | 0.69 | (0.65–0.74) | |
| 1970−1979 | 79 | 1339 | 23.9 | (20.1–25.5) | 0.53 | (0.49–0.57) | |
| 1980+ |
38 |
445 |
11.1 |
(8.2–15.9) |
0.36 |
(0.34–0.39) |
|
|
Age at first exposure (years) |
|
|
|
|
|
|
P<0.001 |
| <20 | 246 | 3662 | 40.6 | (37.8–43.1) | 1.00 | (ref) | |
| 20−29 | 166 | 2604 | 34.5 | (31.0–37.4) | 0.93 | (0.88–0.98) | |
| 30−39 | 92 | 1512 | 30.2 | (25.0–32.8) | 0.82 | (0.77–0.87) | |
| 40−49 | 65 | 952 | 18.2 | (1.2–24.3) | 0.65 | (0.60–0.70) | |
| 50+ |
45 |
550 |
10.7 |
(7.7–15.2) |
0.61 |
(0.56–0.67) |
|
|
Duration of exposure (years) |
|
|
|
|
|
|
P<0.001 |
| <10 | 87 | 1415 | 17.0 | (13.7–20.3) | 1.00 | (ref) | |
| 10−19 | 115 | 2215 | 30.9 | (28.0–33.2) | 1.29 | (1.20–1.39) | |
| 20−29 | 164 | 2739 | 35.6 | (34.7–37.7) | 1.46 | (1.36–1.56) | |
| 30−39 | 162 | 2218 | 44.2 | (43.0–45.3) | 1.62 | (1.51–1.73) | |
| 40+ |
86 |
694 |
48.4 |
(45.8–50.2) |
1.70 |
(1.59–1.83) |
|
|
Mesothelioma type |
|
|
|
|
|
|
P=0.163 |
| Pleural | 301 | 4648 | 22.9 | (14.2–28.6) | 1.00 | (ref) | |
| Peritoneal | 132 | 1919 | 8.2 | (2.2–29.9) | 1.06 | (1.00–1.13) | |
| Pleural+peritoneal | 10 | 94 | 2.9 | (2.9–NE) | 0.92 | (0.77–1.10) | |
| Not specified |
171 |
2620 |
25.5 |
(18.2–28.9) |
1.01 |
(0.95–1.07) |
|
|
Died with asbestosis |
|
|
|
|
|
|
P=0.654 |
| No | 526 | 8286 | 24.9 | (18.2–28.3) | 1.00 | (ref) | |
| Yes | 88 | 994 | 7.7 | (2.2–20.6) | 1.02 | (0.94–1.10) |
Abbreviations: CI=confidence interval; LR test=likelihood-ratio test; NA=not applicable; NE=not estimable; ref=reference category.
Table 3 shows the TRs adjusted for year of first exposure and age at first exposure, and also the TRs from the full model including all variables. Adjusting for year of first exposure and age at first exposure made very little difference when compared with the crude TRs (Table 3 compared with Table 2). The main difference was for duration of exposure, which became of borderline statistical significance (P=0.057) and lost the trend of increasing latency with increasing duration (Table 3). In addition, mesothelioma type became of borderline statistical significance (P=0.076) due to a statistically significant shorter latency for those with pleural and peritoneal mesothelioma compared with those with just pleural mesothelioma (TR=0.83, 95% CI=0.74–0.92).
Table 3. Adjusted time ratios for mesothelioma latency among British asbestos workers (1978–2005), estimated using generalised gamma accelerated failure-time models (N=614).
| Adjusted for age and year of first exposure | Full model (all variables included) | |||||
|---|---|---|---|---|---|---|
|
Characteristic |
Time ratio |
(95% CI) |
LR test |
Time ratio |
(95% CI) |
LR test |
|
Sex |
|
|
P<0.001 |
|
|
P<0.001 |
| Male | 1.00 | (ref) | 1.00 | (ref) | ||
| Female |
1.21 |
(1.11–1.31) |
|
1.29 |
(1.18–1.42) |
|
|
Main smoking status |
|
|
P=0.761 |
|
|
P=0.201 |
| Current | 1.00 | (ref) | 1.00 | (ref) | ||
| Former | 1.01 | (0.97–1.04) | 1.03 | (1.00–1.06) | ||
| Never |
0.99 |
(0.95–1.03) |
|
1.00 |
(0.97–1.04) |
|
|
Main occupation |
|
|
P=0.339 |
|
|
P=0.332 |
| Manufacturing | 1.03 | (0.99–1.07) | 0.98 | (0.95–1.02) | ||
| Removal | 1.00 | (0.96–1.04) | 0.98 | (0.94–1.01) | ||
| Other | 1.01 | (0.96–1.06) | 1.01 | (0.97–1.06) | ||
| Insulation |
1.00 |
(ref) |
|
1.00 |
(ref) |
|
|
Year of first exposure |
|
|
P<0.001 |
|
|
P<0.001 |
| <1940 | 1.00 | (ref) | 1.00 | (ref) | ||
| 1940−1949 | 0.95 | (0.89–1.02) | 1.02 | (0.96–1.08) | ||
| 1950−1959 | 0.84 | (0.79–0.90) | 0.91 | (0.85–0.97) | ||
| 1960−1969 | 0.72 | (0.67–0.78) | 0.78 | (0.72–0.84) | ||
| 1970−1979 | 0.58 | (0.54–0.63) | 0.65 | (0.59–0.72) | ||
| 1980+ |
0.39 |
(0.35–0.42) |
|
0.44 |
(0.40–0.49) |
|
|
Age at first exposure (years) |
|
|
P<0.001 |
|
|
P<0.001 |
| <20 | 1.00 | (ref) | 1.00 | (ref) | ||
| 20−29 | 0.95 | (0.92–0.98) | 0.94 | (0.91–0.97) | ||
| 30−39 | 0.94 | (0.90–0.99) | 0.93 | (0.89–0.98) | ||
| 40−49 | 0.82 | (0.77–0.87) | 0.79 | (0.74–0.84) | ||
| 50+ |
0.82 |
(0.76–0.88) |
|
0.84 |
(0.78–0.90) |
|
|
Duration of exposure (years) |
|
|
P=0.057 |
|
|
P=0.009 |
| <10 | 1.00 | (ref) | 1.00 | (ref) | ||
| 10−19 | 1.09 | (1.03–1.15) | 1.09 | (1.04–1.16) | ||
| 20−29 | 1.06 | (0.99–1.14) | 1.05 | (0.98–1.13) | ||
| 30−39 | 1.08 | (1.00–1.17) | 1.08 | (1.00–1.18) | ||
| 40+ |
1.06 |
(0.97–1.16) |
|
1.07 |
(0.98–1.18) |
|
|
Mesothelioma type |
|
|
P=0.076 |
|
|
P=0.073 |
| Pleural | 1.00 | (ref) | 1.00 | (ref) | ||
| Peritoneal | 1.00 | (0.96–1.04) | 0.99 | (0.96–1.03) | ||
| Pleural+peritoneal | 0.83 | (0.74–0.92) | 0.84 | (0.77–0.93) | ||
| Not specified |
0.99 |
(0.96–1.03) |
|
1.00 |
(0.97–1.03) |
|
|
Died with asbestosis |
|
|
P=0.904 |
|
|
P=0.025 |
| No | 1.00 | (ref) | 1.00 | (ref) | ||
| Yes | 1.00 | (0.95–1.05) | 0.95 | (0.91–0.99) | ||
Abbreviations: CI=confidence interval; LR test=likelihood-ratio test; ref=reference category.
In the full multivariable AFT model shown in Table 3, sex, year of first exposure, age at first exposure, duration of exposure, and whether or not the worker died with asbestosis were all statistically significantly associated with mesothelioma latency. Females tended to have latencies that were, on average, 29% longer than males (TR 1.29, 95% CI 1.18–1.42), which represented around a 10-year difference (adjusted median latency: female=43.7 years, 95% CI=39.7–47.8; male=33.8 years, 95% CI=32.4–35.1). Latency decreased with year of first exposure, such that those first exposed in 1980 or later had 56% shorter latencies than those first exposed before 1940 (TR=0.44, 95% CI=0.40–0.49). However, a decrease was not observed immediately, with no evidence that the latency differed between workers first exposed during 1940 to 1949 and those first exposed before 1940 (TR=1.02, 95% CI=0.96–1.08). Latency also tended to decrease with age at first exposure. This decrease was immediate; those first exposed aged 20 to 29 had, on average, 6% shorter latencies than those first exposed aged less than 20 years (TR=0.94, 95% CI=0.91–0.97). There was strong evidence that duration of exposure was associated with latency (P=0.009). There was an initial increase in latency for those exposed for 10–19 years compared with those exposed for less than 10 years (TR=1.09, 95% CI=1.04–1.16), but there was no statistically significant change after this. There was no indication that death with asbestosis was associated with latency from the univariable (crude) analyses or after adjustment for year of first exposure and age at first exposure; however, there was evidence of an association in the full model (P=0.025; Table 3). Those who died with asbestosis tended to have latencies that were, on average, 5% shorter than those who did not have asbestosis when they died (TR=0.95, 95% CI=0.91–0.99), which represented around a 2-year difference (adjusted median latency: with asbestosis=32.6 years, 95% CI=30.9–34.3; without asbestosis=34.3 years, 95% CI=32.9–35.7). Main smoking status, main occupation, and type of mesothelioma were not statistically significantly associated with mesothelioma latency (all P>0.05; Table 3). Note that, after adjustment for all variables in the full AFT model, the overall adjusted median latency was 34.0 years (95% CI 32.7–35.4 years).
Table 4 shows the TRs by main industry sector, adjusted using the full AFT model. Nearly a quarter (24%) of deaths with mesothelioma were missing industrial sector. Overall, there was no evidence of an association between main industry sector and mesothelioma latency (P=0.114), after adjustment for the other variables of interest. Workers in the building and construction sector tended to have latencies that were around 19% shorter than insulation workers (TR=0.81, 95% CI=0.72–0.90), but this TR was based on a small number of cases (Table 4).
Table 4. Median mesothelioma latency and adjusted time ratios for mesothelioma latency among British asbestos workers (1978–2005), by main industry sector.
| Main industry sector during follow-up | No. of deaths | Person- years at risk | Median latency (years) | (95% CI) | Adj. time ratio | (95% CI) | LR test |
|---|---|---|---|---|---|---|---|
|
Manufacturing |
|
|
|
|
|
|
P=0.114 |
| Textiles | 16 | 246 | 34.0 | (15.5–35.7) | 1.07 | (0.91–1.25) | |
| Asbestos cement mixture, board and pipe | 27 | 405 | 15.9 | (6.6–34.6) | 1.01 | (0.95–1.08) | |
| Asbestos/rubber/resin/bitumen mixtures | 20 | 260 | 17.7 | (4.8–32.2) | 0.95 | (0.88–1.02) | |
| Asbestos board and paper | <5 | NR | NR | NR | NR | NR | |
| Garments | <5 | NR | NR | NR | NR | NR | |
| Insulation and plastering mixes | <5 | NR | NR | NR | NR | NR | |
| Maintenance | 31 | 473 | 17.5 | (10.5–36.4) | 0.96 | (0.91–1.02) | |
| Missing |
103 |
1672 |
26.1 |
(1.2–34.4) |
0.98 |
(0.94–1.02) |
|
| Removal |
200 |
2899 |
19.6 |
(13.9–26.8) |
0.97 |
(0.94–1.06) |
|
|
Other |
|
|
|
|
|
|
|
| Ship building, repair and breaking | 23 | 329 | 8.2 | (8.2–28.6) | 1.05 | (0.98–1.13) | |
| Building and construction | 6 | 71 | 25.5 | (19.7–NE) | 0.81 | (0.72–0.90) | |
| Miscellaneous | 9 | 169 | 29.9 | (15.4–35.8) | 1.08 | (0.97–1.20) | |
| Missing |
44 |
736 |
27.2 |
(6.2–35.4) |
0.98 |
(0.93–1.03) |
|
| Insulation | 129 | 1915 | 20.3 | (2.2–31.3) | 1.00 | (ref) |
Abbreviations: Adj. time ratio=time ratios adjusted for sex, main smoking status, year since first exposure, age at first exposure, duration of exposure, mesothelioma type, and presence of asbestosis estimated using a generalised gamma accelerated failure-time model; CI=confidence interval; LR test=likelihood-ratio test; NE=not estimable; NR=not reported since number of deaths was fewer than five; ref=reference category.
Including all cases of mesothelioma from 1971 increased the median latency to 26.6 years (95% CI=22.0–28.8 years). The results of both analyses were similar when all observation time was included (from 1971) rather than restricting it to start in 1978 at the earliest.
There were 24 cases of mesothelioma that occurred within 10 years of their first occupational exposure to asbestos. Excluding these cases increased the median latency to 30.2 years (95% CI=28.0–32.3 years). The results of the analyses when these cases were excluded were again similar to when they were included.
Discussion
This study used generalised gamma AFT models to investigate the association between the latency period of mesothelioma and indicators of intensity of asbestos exposure, such as occupation, sex, and presence of asbestosis, among a cohort of British asbestos workers.
There was evidence that sex, year of first occupational asbestos exposure, age at first exposure, duration of exposure, and presence of asbestosis were associated with mesothelioma latency in the fully adjusted AFT model. Mesothelioma latency was around 29% longer for females compared with males (95% CI=1.18–1.342), and 5% shorter for those who died with asbestosis compared with those who did not (95% CI=0.91–0.99). Mesothelioma latency tended to be longer for those occupationally exposed to asbestos for 10–19 years compared with those exposed for less than 10 years, but no further increase in latency was observed after this. Finally, latency decreased with both year of first occupational exposure to asbestos, and age at first exposure. There was no evidence of an association between mesothelioma latency and occupation, main smoking status, and mesothelioma type.
The GB Asbestos Survey has a number of strengths. It consists of a large study population followed-up for a long period of time, which is necessary when studying a rare cancer with a long latency period such as mesothelioma. In addition, it is an occupational cohort where all individuals have worked in the asbestos industry, and so the rate of mesothelioma among this cohort will be greater than if using a population-based cohort. Finally, this was a prospective cohort study, which enabled occupation and smoking habits to be collected on each completed questionnaire.
As an occupational cohort of people who work in the asbestos industry, it is likely that the source of asbestos exposure would be of occupational origin for the majority of cases. However, other sources cannot be ruled out. The median latency period for mesothelioma among this cohort was 22.8 years, which is shorter than that found in the review by Lanphear and Buncher (1992) (median 32 years). Cases of mesothelioma with a latency period of less than 10 years are uncommon (Lanphear and Buncher, 1992), and this study observed 24 such cases. This places doubt on the date and, consequently, source of asbestos exposure for these cases. Excluding them produced an estimated median latency of 30.2 years (95% CI=28.0–32.2), which is closer to that expected. However, the results of the AFT models were not substantially different after this exclusion and so including them did not appear to bias the results. The estimated median latency may also be restricted by the duration of follow-up for the study, and further follow-up would likely increase the estimated median latency. This would affect all groups equally and so, even though the estimated median would change, the effect on the comparisons between groups should be minimal.
The ideal when investigating the intensity hypothesis would be to have actual exposure measurements, but these were not available. Occupation is often used as a proxy for exposure intensity due to variations in exposure with occupation; in particular, insulation workers tend to have relatively high levels of asbestos exposure (Williams et al, 2007). Previous analysis of these data has shown that the risk of death with mesothelioma was greatest among insulation workers compared with the other occupations (Harding and Darnton, 2010), as expected. This suggests that occupation should be a reasonable proxy for exposure intensity in this study.
Insulation workers tended to have shorter latencies than manufacturing and ‘other' workers (median latency 20.3, 24.8 and 25.5 years, respectively), but this was not a statistically significant association. This study therefore provided no evidence that mesothelioma latency was associated with occupation. In the literature, insulation workers were consistently observed to have shorter latencies than other occupations (Bianchi et al, 1993, 1997; Bianchi and Bianchi, 2007), but often this was purely descriptive with no formal statistical tests performed (Yeung et al, 1999; Neumann et al, 2001). The occupational classifications used in this study were more broad than those used in two studies that observed a statistically significant association between occupation and latency (Bianchi et al, 1993, 1997). So there would be greater variability in the intensity of exposure within occupational classifications in this study, potentially attenuating any association between occupation and latency. However, even when additionally breaking down occupation by industry sector, no statistically significant association was observed; although there was a large proportion of missing data in this variable and some groups suffered with a small number of cases. In addition, the analysis in the studies that observed a statistically significant association with occupation (Bianchi et al, 1993, 1997) was univariable, and so confounding from other factors cannot be ruled out as a potential explanation.
Previous studies have concentrated on asbestos insulation workers as the main occupational group of interest, but the majority of the cohort (53%), and a third of those who died with mesothelioma, were asbestos removal workers. This is a relatively new occupational group, which was not common until the 1980s, but it now makes up the vast majority of new recruits into the cohort (Harding et al, 2009). It has been suggested that if controls are correctly implemented and protective equipment correctly used, then occupational exposure to asbestos should be minimal among this group (Lange et al, 2006; Williams et al, 2007); hence, if the intensity hypothesis were true, then asbestos removal workers might be expected to have some of the longest latency periods for mesothelioma. However, this was not observed in this study.
The occupational group of an individual could change from one questionnaire to the next, and so the occupation recorded most often was used for individuals with multiple questionnaires. It is possible that the use of ‘main' occupation may have attenuated any potential association. However, the majority of the whole cohort (90%) and of those who died with mesothelioma (82%) did not change occupation during follow-up, and so it is unlikely that any misclassification due to changes in occupation would greatly affect the results.
Finally, asbestos comes in different forms potentially with different carcinogenic potencies (Hodgson and Darnton, 2000; International Agency for Research on Cancer, 2012), and the type of asbestos exposure could vary by occupation. No studies were found that investigated mesothelioma latency and asbestos type, and so its potential impact is unknown.
Investigating latency as an outcome in itself presents challenges, as it is known to vary with age at exposure, duration of follow-up, and mortality due to other causes (Peto, 1985). Adjustment was made for age at first exposure and year of first exposure, which showed the expected decrease in latency with increasing year and age. However, mortality due to other causes is more difficult to take into account. A group with a greater mortality rate or, equivalently, shorter life expectancy would be expected to have a shorter average latency than a group with lower mortality rates; basically, they would not have survived long enough to experience the longer latency periods. Males and people with asbestosis tend to have shorter life expectancies than females and people without asbestosis, respectively, and so this could offer a potential alternative explanation for the associations observed with sex and presence of asbestosis. However, there was no association observed between smoking status and mesothelioma latency. Smokers have a much greater risk of mortality than never smokers, particularly in a population that has also been exposed to asbestos; there is a greater than additive interaction between smoking and asbestos exposure on lung cancer risk (Wraith and Mengersen, 2007). Previous analysis of this cohort found the rate of lung cancer mortality for current smokers was nearly 15 times greater than that compared with never smokers (Frost et al, 2011). The lack of association between mesothelioma latency and smoking status suggests that differences in mortality due to other causes may not be substantially affecting the comparisons of mesothelioma latency in this study, and so it is unlikely that this could fully explain the associations observed with sex and presence of asbestosis.
The association between sex and mesothelioma has not been consistent in the literature (Metintas et al, 1999; Hyland et al, 2007; Haber and Haber, 2011). Haber and Haber (2011) found that women had longer latencies than men, and attributed this to women tending to have lower exposures than men due to their mainly non-occupational asbestos exposure. However, the source of exposure in this study was occupational for both sexes, and so this cannot be an explanation here. There could be heterogeneity in their exposure patterns within the occupation, perhaps in the specific jobs they undertook, and so exposure intensity could still be an explanation for the observed association.
Those with asbestosis at the time of death tended to have shorter latencies than those without asbestosis. As those with asbestosis are thought to have experienced more intense asbestos exposures (Lemen and Dement, 1980; Jamrozik et al, 2011), this appears to support the intensity hypothesis. The association between asbestosis and mesothelioma was statistically significant only after adjustment for all variables. This is probably due to confounding by sex, which is associated with risk of asbestosis (Harding and Darnton, 2010) and mesothelioma latency, disguising the true association in the crude analysis. However, in absolute terms, the association between latency and presence of asbestosis represents a difference in latency of just 2 years, and so this factor does not appear to be as important as sex in determining latency.
Duration of exposure and latency are closely linked—for example, someone with more than 20 years of exposure could not have a latency of less than 20 years. In fact, duration of exposure and latency will be identical while the individual is still ‘exposed', with the alignment between the two only ending when the individual was no longer exposed. The initial increase in latency with increasing duration observed in this study is therefore probably attributable to the alignment between the two.
Median latency was shorter for those with peritoneal mesothelioma compared with those with pleural mesothelioma (median latency 8.2 and 22.9 years, respectively), which was in agreement with that observed in other studies (Chahinian et al, 1982; Neumann et al, 2001; Hyland et al, 2007; Haber and Haber, 2011). However, the median latency, particularly for peritoneal mesothelioma, was subject to a high degree of uncertainty, and the difference was not statistically significant. Type of mesothelioma was ascertained using a combination of the GB Mesothelioma Register, death certificates, and cancer registrations. So there is high potential for misclassification of mesothelioma type, which could at least partially explain the large variability in median latency, and it could attenuate any difference.
There were three main indicators of intensity of asbestos exposure specified in the aims and objectives that were used to judge the strength of support for the intensity hypothesis: sex, presence of asbestosis, and occupation. For both sex and asbestosis, the differences in mesothelioma latency were in the directions expected if the hypothesis was true. However, as discussed above, it was difficult to attribute the sex differences to differences in exposure intensity, and differences in mortality rates cannot be completely ruled out as potentially distorting the association with both sex and asbestosis. Occupation is probably the strongest indicator of intensity of asbestos exposure and has the most consistent association with latency in the literature, but this study found no evidence that insulation workers had shorter latencies than other asbestos workers. Therefore, although there was some evidence supporting the intensity hypothesis due to the associations with sex and asbestosis, sufficient doubt remains.
Acknowledgments
This analysis was undertaken as part of a Masters course at the London School of Hygiene and Tropical Medicine. Participation in the course and preparation of the manuscript was funded by the Health and Safety Laboratory. The GB Asbestos Survey is funded by the Health and Safety Executive. I thank the staff at the Health and Safety Laboratory who work on the GB Asbestos Survey. In particular, I thank Anne-Helen Harding of the Health and Safety Laboratory and Andrew Darnton of the Health and Safety Executive for their support, and Clare Gilham of the London School of Hygiene and Tropical Medicine for her insightful supervision during the project. I also thank the staff at the HSCIC, GROS, the occupational physicians, and the asbestos workers for their support.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Bianchi C, Bianchi T. Malignant mesothelioma: global incidence and relationship with asbestos. Ind Health. 2007;45:379–387. doi: 10.2486/indhealth.45.379. [DOI] [PubMed] [Google Scholar]
- Bianchi C, Brollo A, Ramani L, Zuch C. Asbestos-related mesothelioma in Monfalcone, Italy. Am J Ind Med. 1993;24:149–160. doi: 10.1002/ajim.4700240203. [DOI] [PubMed] [Google Scholar]
- Bianchi C, Giarelli L, Grandi G, Brollo A, Ramani L, Zuch C. Latency periods in asbestos-related mesothelioma of the pleura. Eur J Cancer Prev. 1997;6:162–166. [PubMed] [Google Scholar]
- Cancer Research UK 2012MesotheliomaAvailable from http://cancerhelp.cancerresearchuk.org/type/mesothelioma/ .
- Chahinian AP, Pajak TF, Holland JF, Norton L, Ambinder RM, Mandel EM. Diffuse malignant mesothelioma. Prospective evaluation of 69 patients. Ann Intern Med. 1982;96:746–755. doi: 10.7326/0003-4819-96-6-746. [DOI] [PubMed] [Google Scholar]
- Cleves MA, Gould WW, Gutierrez RG.2004An Introduction to Survival Analysis Using StataRevised editionStata Press: Texas, USA [Google Scholar]
- Frost G, Darnton A, Harding AH. The effect of smoking on the risk of lung cancer mortality for asbestos workers in Great Britain (1971-2005) Ann Occup Hyg. 2011;55:239–247. doi: 10.1093/annhyg/meq089. [DOI] [PubMed] [Google Scholar]
- Haber SE, Haber JM. Malignant mesothelioma: a clinical study of 238 cases. Ind Health. 2011;49:166–172. doi: 10.2486/indhealth.ms1147. [DOI] [PubMed] [Google Scholar]
- Harding A-H, Darnton A, Wegerdt J, McElvenny D. Mortality among British asbestos workers undergoing regular medical examinations (1971–2005) Occup Environ Med. 2009;66:487–495. doi: 10.1136/oem.2008.043414. [DOI] [PubMed] [Google Scholar]
- Harding A-H, Darnton AJ. Asbestosis and mesothelioma mortality among British asbestos workers (1971-2005) Am J Ind Med. 2010;53:1070–1080. doi: 10.1002/ajim.20844. [DOI] [PubMed] [Google Scholar]
- Harding A-H, Frost G. HSE Research Report RR730. Health & Safety Executive: Bootle, UK; 2010. The Asbestos Survey: mortality among asbestos workers 1971-2005. [Google Scholar]
- Harding A-H, Wegerdt J. HSE Research Report HSL/2007/05. Health & Safety Executive: Bootle, UK; 2007. Asbestos Workers Database: Summary Statistics. [Google Scholar]
- Health and Safety Executive 2013a. Data sources: Death certificates as a source of deaths from asbestos-related and other occupational lung diseases. Available from http://www.hse.gov.uk/statistics/sources.htm .
- Health and Safety Executive 2013b. Mesothelioma: Mesothelioma in Great Britain 1968-2010. Available from http://www.hse.gov.uk/statistics/causdis/mesothelioma/index.htm .
- Hodgson JT, Darnton A. The quantitative risks of mesothelioma and lung cancer in relation to asbestos exposure. Ann Occup Hyg. 2000;44:565–601. [PubMed] [Google Scholar]
- Hyland RA, Ware S, Johnson AR, Yates DH. Incidence trends and gender differences in malignant mesothelioma in New South Wales, Australia. Scand J Work Environ Health. 2007;33:286–292. doi: 10.5271/sjweh.1145. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer Asbestos (Chrysotile, Amosite, Crocidolite, Tremolite, Actinolite, and Anthophyllite) IARC Monogr Eval Carcinog Risks Hum. 2012;100C:1–309. [Google Scholar]
- Jamrozik E, de Klerk N, Musk AW. Asbestos-related disease. Intern Med J. 2011;41:372–380. doi: 10.1111/j.1445-5994.2011.02451.x. [DOI] [PubMed] [Google Scholar]
- Lange JH, Sites SLM, Mastrangelo G, Thomulka KW. Exposure to airborne asbestos during abatement of ceiling material, window caulking, floor tile, and roofing material. Bull Environ Contam Toxicol. 2006;77:718–722. doi: 10.1007/s00128-006-1122-8. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Buncher CR. Latent period for malignant mesothelioma of occupational origin. J Occup Med. 1992;34:718–721. [PubMed] [Google Scholar]
- Lemen RA, Dement JMW, J K. Epidemiology of asbestos-related disease. Environ Health Perspect. 1980;34:1–11. doi: 10.1289/ehp.80341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J, Gravelsons B, Hawes W, Jakubowski S, Kent A, Macnair A, Michaels D, Morton A, Sanders D, Towell P, Whiting A, Widdows J, Williams A.2004UK asbestos—the definitive guide The ActuaryAvailable at http://www.theactuary.com/archive/old-articles/part-2/uk-asbestos—-the-definitive-guide/ .
- Marinaccio A, Binazzi A, Cauzillo G, Cavone D, Zotti RD, Ferrante P, Gennaro V, Gorini G, Menegozzo M, Mensi C, Merler E, Mirabelli D, Montanaro F, Musti M, Pannelli F, Romanelli A, Scarselli A, Tumino R, Italian Mesothelioma Register (ReNaM) Working Group Analysis of latency time and its determinants in asbestos related malignant mesothelioma cases of the Italian register. Eur J Cancer. 2007;43:2722–2728. doi: 10.1016/j.ejca.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Metintas M, Ozdemir N, Hillerdal G, Uçgun I, Metintas S, Baykul C, Elbek O, Mutlu S, Kolsuz M. Environmental asbestos exposure and malignant pleural mesothelioma. Respir Med. 1999;93:349–355. doi: 10.1016/s0954-6111(99)90318-9. [DOI] [PubMed] [Google Scholar]
- Neumann V, Günthe S, Mülle KM, Fischer M. Malignant mesothelioma—German mesothelioma register 1987-1999. Int Arch Occup Environ Health. 2001;74:383–395. doi: 10.1007/s004200100240. [DOI] [PubMed] [Google Scholar]
- Oracle Solutions 2013History of asbestos law and regulationsAvailable from http://www.oracleasbestos.com/a-complete-guide-to-the-history-of-asbestos-law-and-regulations/ .
- Peto J.1985Some problems in dose-response estimation in cancer epidemiology In Methods for estimating risk of chemical injury: human and non-human biota and ecosystems, Vouk VB, Butler GC, Hoel DG, Peakall D (eds) pp361–380.Wiley and Sons: New York [Google Scholar]
- StataCorp . Stata Statistical Software: Release 12.1. StataCorp LP.: College Station, TX; 2012. [Google Scholar]
- Tan E, Warren N. HSE Research Report RR876. Health & Safety Executive: Bootle, UK; 2011. Mesothelioma mortality in Great Britain: The revised risk and two-stage clonal expansion models. [Google Scholar]
- Williams PRD, Phelka AD, Paustenbach DJ. A review of historical exposures to asbestos among skilled craftsmen (1940-2006) J Toxicol Environ Health B Crit Rev. 2007;10:319–377. doi: 10.1080/10937400601034191. [DOI] [PubMed] [Google Scholar]
- Wraith D, Mengersen K. Assessing the combined effect of asbestos exposure and smoking on lung cancer: a Bayesian approach. Stat Med. 2007;26:1150–1169. doi: 10.1002/sim.2602. [DOI] [PubMed] [Google Scholar]
- Yeung P, Rogers A, Johnson A. Distribution of mesothelioma cases in different occupational groups and industries in Australia, 1979-1995. Appl Occup Environ Hyg. 1999;14:759–767. doi: 10.1080/104732299302189. [DOI] [PubMed] [Google Scholar]