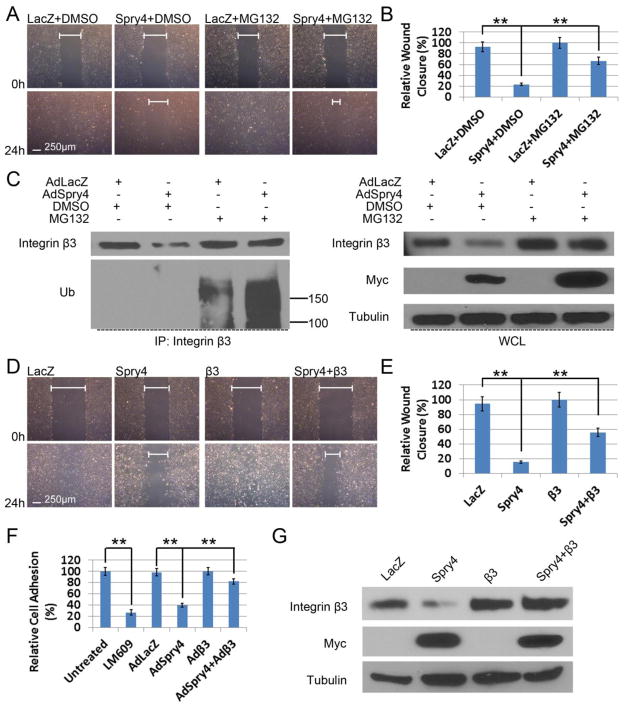
Fig. 6. Spry4 regulates human endothelial cell migration and adhesion on VTN via modulating integrin β3 protein levels.
(A) MG132 rescues migration of HUVECs overexpressing Spry4. Confluent HUVECs grown on VTN were transduced with AdLacZ or AdSpry4 and subjected to wound healing assays with DMSO or MG132 (5 μM) 24 h later. Confluent monolayers were pre-treated with mitomycin C (10 μg/ml) for 20 min, scratched, and photographed at the indicated times. (B) Quantification of relative wound closure; n = 6; data are means ± S.D. ** p < 0.01. (C) HUVECs were transduced with the indicated adenoviruses and treated with MG132 as described in A, and analyzed by immunoprecipitation and immunoblotting. Representative immunoblots are shown. Equal amount of cell lysate were loaded for all samples. (D) Confluent HUVECs grown on VTN were transduced with adenoviruses as indicated and subjected to wound healing assays 24 h later as described in A. (E) Quantification of relative wound closure; n = 6; data are means ± S.D. ** p < 0.01. (F) Overexpression of exogenous integrin β3 rescues inhibition of cell adhesion by Spry4. HUVECs were transduced with adenoviruses as indicated and subjected to adhesion assays on VTN (0.5 μg/ml) 24 h later. Cell adhesion was quantified relative to untreated control; n = 3; data are means ± S.D. ** p < 0.01. The integrin αVβ3-blocking antibody LM609 was used as a positive control. (G) Cell lysates of the transduced HUVECs subjected to scratch assays were analyzed by immunoblotting to confirm exogenous integrin β3 expression. Representative immunoblots are shown. Equal amount of cell lysate were loaded for all samples.