Abstract
In the title salt [systematic name: 3-(1H-imidazol-1-yl)propanaminium 2,4,6-trinitrophenolate], C6H12N3 +·C6H2N3O7 −, there are five independent cation–anion pairs (A, B, C, D, E) in the asymmetric unit. In the cation, the ammonium group is protonated with the aminopropyl group nearly at right angles to the mean plane of the imidazole ring showing C—N—C—C torsion angles ranging from 79.6 (2) to 99.79 (19)° in the five cations. The nitro groups in the anion are twisted from the benzene mean plane with maximum dihedral angles subtended by nitro substituents ortho to the phenolate O atom of 26.0 (2) and 37.3 (7) (A), 28.9 (5) and 35.3 (1) (B), 34.7 (7) and 36.9 (7) (C), 14.7 (4) and 36.9 (2) (D) and 33.1 (1) and 35.4 (3)° (E). In contrast, the nitro groups in the para positions lie much closer to the aromatic ring plane, subtending dihedral angles of 1.8 (3) (A), 3.5 (3) (B), 6.03 (C), 2.1 (3) (D) and 7.7 (1)° (E). Disorder is observed for one O atom of an ortho nitro group in anion D with an occupancy ratio of 0.53 (5):0.47 (5). In the crystal, N—H⋯O cation–anion and N—H⋯N cation–cation hydrogen bonds are observed, linking the ions into chains along [010]. In addition, weak C—H⋯O cation–anion interactions occur.
Related literature
For pharmacological properties of imidazole compounds, see: Lombardino & Wiseman (1974 ▶). For applications of substituted imidazoles, see: Maier et al. (1989a
▶,b
▶). For imidazole derivatives as anticancer agents, see: Krezel (1998 ▶). For electrostatic or hydrogen-bonding interactions in picric acid charge-transfer complexes, see: In et al. (1997 ▶). For imidazolium-based cation picrate salts as good candidates for energetic ionic salts, see: Jin et al. (2005 ▶). For the crystal structure of imidazolium picrate, see: Soriano-García et al. (1990 ▶) and for the structures of picrates of some other imidazole derivatives, see: Du & Zhao (2003 ▶); Dutkiewicz et al. (2011 ▶); MacDonald et al. (2005 ▶); Nardelli et al. (1987 ▶); Pi et al. (2009 ▶). For standard bond lengths, see: Allen et al. (1987 ▶).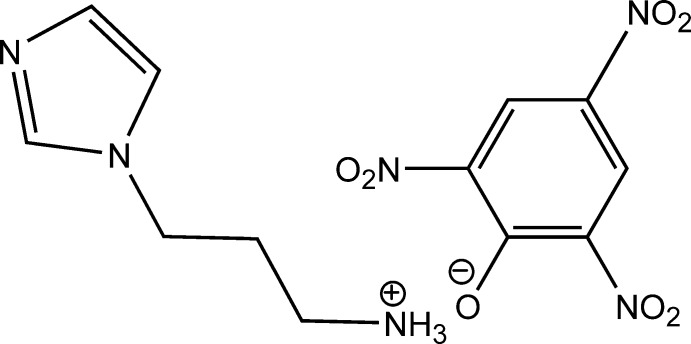
Experimental
Crystal data
C6H12N3 +·C6H2N3O7 −
M r = 354.29
Monoclinic,
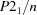
a = 11.98275 (18) Å
b = 38.5234 (6) Å
c = 16.4239 (2) Å
β = 94.1970 (14)°
V = 7561.2 (2) Å3
Z = 20
Cu Kα radiation
μ = 1.13 mm−1
T = 173 K
0.21 × 0.17 × 0.08 mm
Data collection
Agilent Xcalibur (Eos, Gemini) diffractometer
Absorption correction: multi-scan (CrysAlis PRO and CrysAlis RED; Agilent, 2012 ▶) T min = 0.870, T max = 1.000
52087 measured reflections
14795 independent reflections
12167 reflections with I > 2σ(I)
R int = 0.032
Refinement
R[F 2 > 2σ(F 2)] = 0.042
wR(F 2) = 0.116
S = 1.02
14795 reflections
1197 parameters
12 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.51 e Å−3
Δρmin = −0.29 e Å−3
Data collection: CrysAlis PRO (Agilent, 2012 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis RED (Agilent, 2012 ▶); program(s) used to solve structure: SUPERFLIP (Palatinus & Chapuis, 2007 ▶); program(s) used to refine structure: SHELXL2012 (Sheldrick, 2008 ▶); molecular graphics: OLEX2 (Dolomanov et al., 2009 ▶); software used to prepare material for publication: OLEX2.
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S1600536813025646/sj5352sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813025646/sj5352Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813025646/sj5352Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N6A—H6AA⋯O3E i | 0.85 (2) | 2.06 (2) | 2.8943 (18) | 167.5 (18) |
| N6A—H6AB⋯O3D ii | 0.91 (2) | 2.08 (2) | 2.8671 (18) | 143.1 (16) |
| N6A—H6AB⋯O4D ii | 0.91 (2) | 2.25 (2) | 2.961 (2) | 134.6 (15) |
| N6A—H6AC⋯N4C | 0.95 (2) | 1.87 (2) | 2.8157 (19) | 172.5 (18) |
| N6B—H6BA⋯O3B iii | 0.92 (2) | 2.117 (19) | 2.8728 (17) | 138.5 (15) |
| N6B—H6BA⋯O4B iii | 0.92 (2) | 2.268 (19) | 3.006 (2) | 136.6 (15) |
| N6B—H6BB⋯O3B | 0.89 (2) | 2.00 (2) | 2.8502 (17) | 161.0 (19) |
| N6B—H6BC⋯N4A iv | 0.92 (2) | 1.88 (2) | 2.7988 (19) | 173.4 (17) |
| N6C—H6CA⋯O2C v | 0.85 (2) | 2.305 (18) | 2.8334 (19) | 120.9 (15) |
| N6C—H6CA⋯O3C v | 0.85 (2) | 2.10 (2) | 2.8944 (18) | 155.4 (17) |
| N6C—H6CB⋯O3A vi | 0.89 (2) | 2.174 (19) | 2.9145 (18) | 140.2 (16) |
| N6C—H6CB⋯O4A vi | 0.89 (2) | 2.270 (19) | 2.986 (2) | 137.3 (15) |
| N6C—H6CC⋯N4D vi | 0.92 (2) | 1.91 (2) | 2.8179 (19) | 167.6 (17) |
| N6D—H6DA⋯O2A vii | 0.89 (2) | 2.361 (19) | 2.9631 (19) | 124.9 (15) |
| N6D—H6DA⋯O3A vii | 0.89 (2) | 2.06 (2) | 2.8340 (17) | 145.0 (16) |
| N6D—H6DB⋯O3C vi | 0.89 (2) | 2.16 (2) | 2.9171 (17) | 142.0 (17) |
| N6D—H6DB⋯O4C vi | 0.89 (2) | 2.27 (2) | 2.9323 (19) | 130.5 (16) |
| N6D—H6DC⋯N4E | 0.89 (2) | 1.91 (2) | 2.7932 (19) | 174.0 (17) |
| N6E—H6EA⋯O2D viii | 0.91 (2) | 2.32 (2) | 2.973 (2) | 128.7 (17) |
| N6E—H6EA⋯O3D viii | 0.91 (2) | 2.06 (2) | 2.8527 (18) | 145.4 (18) |
| N6E—H6EB⋯O3E | 0.87 (2) | 2.19 (2) | 2.9056 (18) | 139.6 (17) |
| N6E—H6EB⋯O4E | 0.87 (2) | 2.33 (2) | 3.010 (2) | 135.3 (16) |
| N6E—H6EC⋯N4B viii | 0.93 (2) | 1.85 (2) | 2.7750 (19) | 174.0 (19) |
| C8A—H8A⋯O2B ix | 0.95 | 2.46 | 3.0887 (19) | 123 |
| C9A—H9A⋯O5E | 0.95 | 2.43 | 3.243 (2) | 144 |
| C12A—H12B⋯O7A ix | 0.99 | 2.46 | 3.313 (2) | 145 |
| C9B—H9B⋯O5B i | 0.95 | 2.37 | 3.224 (2) | 150 |
| C12B—H12D⋯O7C vi | 0.99 | 2.47 | 3.335 (2) | 145 |
| C8C—H8C⋯O5C vi | 0.95 | 2.33 | 3.194 (2) | 152 |
| C9C—H9C⋯O2E i | 0.95 | 2.49 | 3.105 (2) | 123 |
| C7D—H7D⋯O5A vi | 0.95 | 2.35 | 3.233 (2) | 155 |
| C11D—H11H⋯O6A vi | 0.99 | 2.55 | 3.421 (2) | 147 |
| C12D—H12G⋯O6B vii | 0.99 | 2.43 | 3.238 (2) | 139 |
| C9E—H9E⋯O5D ii | 0.95 | 2.27 | 3.162 (6) | 156 |
| C9E—H9E⋯O5DA ii | 0.95 | 2.56 | 3.357 (17) | 141 |
| C12E—H12I⋯O6E viii | 0.99 | 2.34 | 3.186 (2) | 142 |
Symmetry codes: (i) 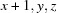 ; (ii)
; (ii) 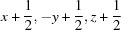 ; (iii)
; (iii) 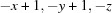 ; (iv)
; (iv) 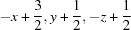 ; (v)
; (v) 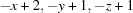 ; (vi)
; (vi) 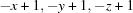 ; (vii)
; (vii) 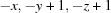 ; (viii)
; (viii) 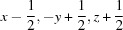 ; (ix)
; (ix) 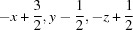 .
.
Acknowledgments
TSY thanks the UOM for research facilities and is also grateful to the Principal, Maharani’s Science College for Women, Mysore, for giving permission to do research. JPJ acknowledges the NSF–MRI program (grant No. CHE-1039027) for funds to purchase the X-ray diffractometer.
supplementary crystallographic information
1. Comment
Compounds with an imidazole ring system have many pharmacological properties and play important roles in biochemical processes (Lombardino & Wiseman, 1974). Many imidazole derivatives are characterized as inhibitors of fungicides and herbicides, plant growth regulators and therapeutic agents (Maier et al., 1989a,b). Imidazole derivatives are also used as potential anticancer agents (Krezel, 1998) and display a broad spectrum of pharmacological activities. It is well known that picric acid forms charge transfer molecular complexes with a number of aromatic compounds such as aromatic amines through electrostatic or hydrogen bonding interactions (In et al., 1997). Picric acid is a polynitrogen compound with explosive character and imidazolium-based cation picrate salts are good candidates for energetic ionic salts (Jin et al., 2005).
The crystal structures of some imidazolium picrates have been reported, for instance imidazolium picrate itself (Soriano-García et al., 1990), also two solvates (hydrate and ethanolate) of 2-aminohistamine dipicrate (Nardelli et al., 1987), 4-hydroxy methylimidazolium picrate (Du & Zhao, 2003), two polymorphs of betaine bis(diimidazolium) dipicrate (MacDonald et al., 2005) and 3-benzyl-1-methyl-imidazolium picrate (Pi et al., 2009). Recently, we have reported the crystal structure of 2-methylimidazolium picrate (Dutkiewicz et al., 2011).
In view of the importance of imidazoles, this paper reports the crystal structure of the title salt, C6H12N3+ . C6H2N3O7-, (I).
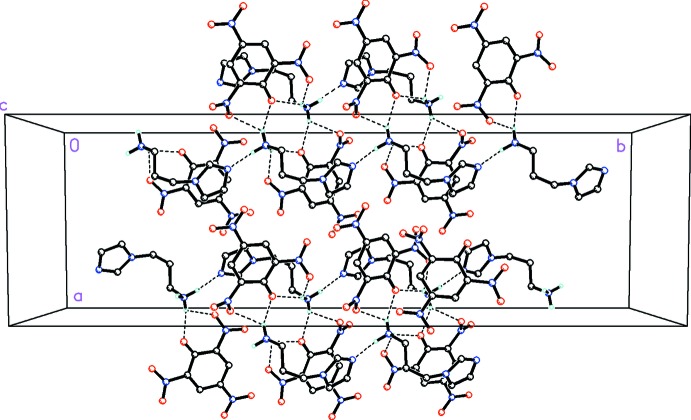
In (I), there are five cation-anion independent pairs (A, B, C, D, E) in the asymmetric unit. For clarity only the C cation-anion pair is displayed in Fig. 1. In the cation the ammonium group is protonated with the aminopropyl group nearly at right angles to the imidazole ring showing C/N/C/C torsion angles ranging from 79.6 (2)° to 99.79 (19)° in the five cations. Bond lengths are in normal ranges (Allen et al., 1987). The nitro groups in the anion are twisted from the benzene mean plane with maximum dihedral angles subtended by nitro substituents ortho to phenolate oxygen of 26.0 (2)°, 37,3(7)° (A); 28,9(5)°, 35.3 (1)° (B); 34.7 (7)°, 36.9 (7)° (C); 14.7 (4)°, 36.9 (2)° (D); 33.1 (1)°, 35.4 (3)° (E). In contrast the nitro groups in the para positions lie much closer to the aromatic ring plane with angles 1.8 (3)° (A); 3.5 (3)° (B); 6.03° (C); 2.1 (3)° (D); 7.7 (1)° (E), respectively. Disorder is observed in atom O5D, an ortho nitro group in anion D, with an occupancy ratio of 0.53 (5):0.47 (5). In the crystal, N—H···O cation-anion and N—H···N cation-cation hydrogen bonds (Table 1) are observed linking the ions into chains along [0 1 0] (Fig. 2). In addition, weak C—H···O cation-anion intermolecular interactions also contribute to packing stability.
2. Experimental
Commercially available 1-(3-aminopropyl)imidazole (0.5 g, 3.99 mmol) and picric acid (1.19 g, 3.99 mmol) were dissolved in 10 ml of ethanol and stirred for 5 minutes at room temperature. After 30 mins, crystals were formed on evaporation of ethanol (m.p.: 370–373 K).
3. Refinement
H6AA, H6AB, H6AC, H6BA, H6BB, H6BC, H6CA, H6CB, H6CC, H6DA, H6DB, H6DC, H6EA, H6EB, H6EC, were located by a difference map and refined isotropically. All of the remaining H atoms were placed in their calculated positions and then refined using the riding model with Atom—H lengths of 0.95Å (aromatic) or 0.99Å (CH2). Isotropic displacement parameters for these atoms were set to 1.2 (CH, CH2) times Ueq of the parent atom. Disorder is observed in atom O5D of anion D with an occupancy ratio of 0.53 (5):0.47 (5).
Figures
Fig. 1.
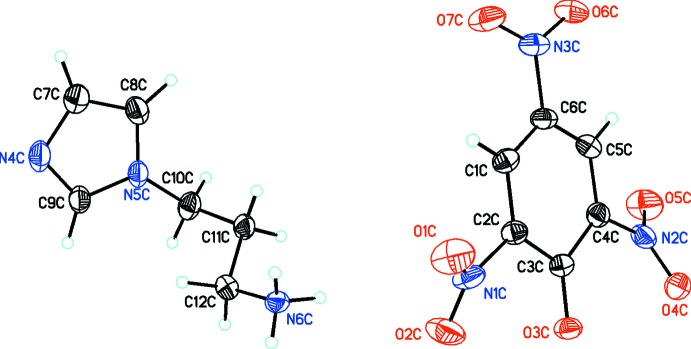
ORTEP drawing of one of five independent cation (C6H12N3+ ) and anion (C6H2N3O7-) pairs (C) in the asymmetric unit of (I), for clarity, showing the atom labeling scheme and 30% probability displacement ellipsoids.
Fig. 2.
Molecular packing for (I) viewed along the c axis. Dashed lines indicate N—H···O cation–anion and N—H···N cation-cation hydrogen bonds, dahed lines, linking the ions into chains along [0 1 0]. Hydrogen atoms not involved in hydrogen bonding are omitted for clarity.
Crystal data
| C6H12N3+·C6H2N3O7− | F(000) = 3680 |
| Mr = 354.29 | Dx = 1.556 Mg m−3 |
| Monoclinic, P21/n | Cu Kα radiation, λ = 1.54184 Å |
| a = 11.98275 (18) Å | Cell parameters from 17325 reflections |
| b = 38.5234 (6) Å | θ = 3.4–72.3° |
| c = 16.4239 (2) Å | µ = 1.13 mm−1 |
| β = 94.1970 (14)° | T = 173 K |
| V = 7561.2 (2) Å3 | Irregular, clear yellow |
| Z = 20 | 0.21 × 0.17 × 0.08 mm |
Data collection
| Agilent Xcalibur (Eos, Gemini) diffractometer | 14795 independent reflections |
| Radiation source: Enhance (Cu) X-ray Source | 12167 reflections with I > 2σ(I) |
| Detector resolution: 16.0416 pixels mm-1 | Rint = 0.032 |
| ω scans | θmax = 72.5°, θmin = 3.5° |
| Absorption correction: multi-scan (CrysAlis PRO and CrysAlis RED; Agilent, 2012) | h = −9→14 |
| Tmin = 0.870, Tmax = 1.000 | k = −47→46 |
| 52087 measured reflections | l = −20→20 |
Refinement
| Refinement on F2 | Hydrogen site location: mixed |
| Least-squares matrix: full | H atoms treated by a mixture of independent and constrained refinement |
| R[F2 > 2σ(F2)] = 0.042 | w = 1/[σ2(Fo2) + (0.0585P)2 + 2.3598P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.116 | (Δ/σ)max = 0.001 |
| S = 1.02 | Δρmax = 0.51 e Å−3 |
| 14795 reflections | Δρmin = −0.29 e Å−3 |
| 1197 parameters | Extinction correction: SHELXL, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 12 restraints | Extinction coefficient: 0.000142 (14) |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| O1A | 0.06660 (12) | 0.70857 (4) | 0.28122 (10) | 0.0543 (4) | |
| O2A | 0.03780 (11) | 0.68796 (4) | 0.40019 (10) | 0.0477 (4) | |
| O3A | 0.11234 (9) | 0.62090 (3) | 0.40908 (7) | 0.0296 (3) | |
| O4A | 0.21360 (12) | 0.56043 (3) | 0.37863 (10) | 0.0491 (4) | |
| O5A | 0.39119 (12) | 0.56511 (4) | 0.39119 (12) | 0.0636 (5) | |
| O6A | 0.55513 (10) | 0.65993 (4) | 0.25325 (8) | 0.0417 (3) | |
| O7A | 0.46158 (11) | 0.70758 (4) | 0.23205 (9) | 0.0446 (3) | |
| N1A | 0.09133 (12) | 0.68950 (4) | 0.33963 (10) | 0.0329 (3) | |
| N2A | 0.29916 (12) | 0.57781 (4) | 0.37670 (9) | 0.0321 (3) | |
| N3A | 0.47021 (12) | 0.67773 (4) | 0.25701 (9) | 0.0318 (3) | |
| C1A | 0.27832 (13) | 0.68191 (4) | 0.29857 (10) | 0.0259 (3) | |
| H1A | 0.2729 | 0.7046 | 0.2761 | 0.031* | |
| C2A | 0.19050 (12) | 0.66773 (4) | 0.33624 (10) | 0.0245 (3) | |
| C3A | 0.18956 (12) | 0.63312 (4) | 0.37119 (9) | 0.0224 (3) | |
| C4A | 0.29093 (13) | 0.61429 (4) | 0.35572 (9) | 0.0246 (3) | |
| C5A | 0.38185 (13) | 0.62869 (4) | 0.32185 (9) | 0.0260 (3) | |
| H5A | 0.4482 | 0.6155 | 0.3178 | 0.031* | |
| C6A | 0.37572 (13) | 0.66236 (4) | 0.29393 (9) | 0.0260 (3) | |
| O1B | 0.41038 (10) | 0.62930 (3) | 0.10159 (8) | 0.0377 (3) | |
| O2B | 0.50153 (9) | 0.58259 (3) | 0.13563 (9) | 0.0385 (3) | |
| O3B | 0.37850 (9) | 0.52841 (3) | 0.06265 (7) | 0.0261 (2) | |
| O4B | 0.25989 (12) | 0.47144 (3) | 0.10774 (9) | 0.0432 (3) | |
| O5B | 0.08374 (12) | 0.48134 (4) | 0.08146 (10) | 0.0571 (4) | |
| O6B | −0.05515 (10) | 0.58211 (4) | 0.20725 (8) | 0.0392 (3) | |
| O7B | 0.04050 (10) | 0.62992 (3) | 0.20991 (8) | 0.0386 (3) | |
| N1B | 0.02960 (11) | 0.59876 (4) | 0.19519 (8) | 0.0299 (3) | |
| N2B | 0.17976 (12) | 0.49122 (4) | 0.10008 (9) | 0.0331 (3) | |
| N3B | 0.41401 (11) | 0.59841 (3) | 0.11880 (8) | 0.0243 (3) | |
| C1B | 0.22192 (12) | 0.59790 (4) | 0.15207 (9) | 0.0232 (3) | |
| H1B | 0.2297 | 0.6219 | 0.1648 | 0.028* | |
| C2B | 0.12167 (13) | 0.58070 (4) | 0.16165 (9) | 0.0255 (3) | |
| C3B | 0.10926 (13) | 0.54597 (4) | 0.14262 (9) | 0.0253 (3) | |
| H3B | 0.0402 | 0.5345 | 0.1490 | 0.030* | |
| C4B | 0.19756 (13) | 0.52809 (4) | 0.11443 (9) | 0.0249 (3) | |
| C5B | 0.30432 (12) | 0.54352 (4) | 0.09843 (9) | 0.0209 (3) | |
| C6B | 0.30933 (12) | 0.57958 (4) | 0.12386 (9) | 0.0214 (3) | |
| O1C | 0.93330 (11) | 0.49777 (4) | 0.71717 (9) | 0.0459 (3) | |
| O2C | 0.95844 (11) | 0.51289 (4) | 0.59358 (10) | 0.0504 (4) | |
| O3C | 0.85836 (9) | 0.57635 (3) | 0.56438 (7) | 0.0274 (2) | |
| O4C | 0.70931 (11) | 0.63065 (3) | 0.55889 (8) | 0.0392 (3) | |
| O5C | 0.54488 (11) | 0.60950 (4) | 0.52653 (10) | 0.0488 (4) | |
| O6C | 0.42608 (9) | 0.52706 (3) | 0.72080 (8) | 0.0357 (3) | |
| O7C | 0.53457 (10) | 0.48321 (3) | 0.75141 (8) | 0.0400 (3) | |
| N1C | 0.90358 (11) | 0.51276 (4) | 0.65333 (9) | 0.0307 (3) | |
| N2C | 0.64000 (12) | 0.60737 (4) | 0.56060 (9) | 0.0306 (3) | |
| N3C | 0.51685 (11) | 0.51198 (4) | 0.72106 (8) | 0.0287 (3) | |
| C1C | 0.71259 (13) | 0.51435 (4) | 0.68752 (9) | 0.0237 (3) | |
| H1C | 0.7274 | 0.4937 | 0.7180 | 0.028* | |
| C2C | 0.79609 (12) | 0.53034 (4) | 0.64799 (9) | 0.0230 (3) | |
| C3C | 0.78325 (12) | 0.56224 (4) | 0.60164 (9) | 0.0217 (3) | |
| C4C | 0.67031 (13) | 0.57559 (4) | 0.60354 (9) | 0.0243 (3) | |
| C5C | 0.58477 (13) | 0.55929 (4) | 0.63995 (9) | 0.0255 (3) | |
| H5C | 0.5116 | 0.5689 | 0.6361 | 0.031* | |
| C6C | 0.60648 (12) | 0.52896 (4) | 0.68195 (9) | 0.0241 (3) | |
| O1D | 0.43842 (13) | 0.41609 (4) | 0.17945 (13) | 0.0753 (6) | |
| O2D | 0.45803 (12) | 0.38759 (4) | 0.06991 (9) | 0.0483 (4) | |
| O3D | 0.39104 (9) | 0.32112 (3) | 0.09394 (8) | 0.0321 (3) | |
| O4D | 0.27366 (16) | 0.26401 (4) | 0.11869 (13) | 0.0756 (6) | |
| O5D | 0.1088 (5) | 0.2689 (2) | 0.132 (2) | 0.074 (6) | 0.53 (5) |
| O5DA | 0.1161 (9) | 0.2685 (2) | 0.1739 (14) | 0.051 (3) | 0.47 (5) |
| O6D | −0.04727 (10) | 0.37181 (4) | 0.23998 (8) | 0.0417 (3) | |
| O7D | 0.04676 (10) | 0.41989 (3) | 0.24429 (8) | 0.0392 (3) | |
| N1D | 0.41073 (12) | 0.39325 (4) | 0.13245 (10) | 0.0335 (3) | |
| N2D | 0.19780 (13) | 0.28171 (4) | 0.13810 (9) | 0.0346 (3) | |
| N3D | 0.03733 (11) | 0.38876 (4) | 0.22938 (8) | 0.0310 (3) | |
| C1D | 0.22787 (13) | 0.38939 (4) | 0.18260 (10) | 0.0259 (3) | |
| H1D | 0.2349 | 0.4132 | 0.1972 | 0.031* | |
| C2D | 0.31297 (13) | 0.37241 (4) | 0.14767 (10) | 0.0246 (3) | |
| C3D | 0.31268 (12) | 0.33593 (4) | 0.12588 (9) | 0.0237 (3) | |
| C4D | 0.21039 (13) | 0.31897 (4) | 0.14756 (9) | 0.0258 (3) | |
| C5D | 0.12236 (13) | 0.33613 (4) | 0.17907 (10) | 0.0271 (3) | |
| H5D | 0.0559 | 0.3239 | 0.1889 | 0.033* | |
| C6D | 0.13078 (13) | 0.37097 (4) | 0.19620 (10) | 0.0266 (3) | |
| O1E | 0.08024 (11) | 0.32801 (3) | 0.38651 (11) | 0.0522 (4) | |
| O2E | 0.00509 (10) | 0.27786 (3) | 0.36309 (9) | 0.0387 (3) | |
| O3E | 0.14077 (9) | 0.23016 (3) | 0.45016 (7) | 0.0273 (2) | |
| O4E | 0.28681 (12) | 0.17609 (3) | 0.42987 (9) | 0.0453 (3) | |
| O5E | 0.45292 (11) | 0.19515 (4) | 0.46414 (11) | 0.0559 (4) | |
| O6E | 0.56176 (10) | 0.29016 (4) | 0.29897 (8) | 0.0429 (3) | |
| O7E | 0.45539 (11) | 0.33582 (4) | 0.29169 (8) | 0.0457 (3) | |
| N1E | 0.08694 (11) | 0.29654 (3) | 0.37717 (8) | 0.0274 (3) | |
| N2E | 0.35637 (12) | 0.19938 (4) | 0.43450 (10) | 0.0344 (3) | |
| N3E | 0.47330 (12) | 0.30510 (4) | 0.30897 (8) | 0.0337 (3) | |
| C1E | 0.28020 (13) | 0.30019 (4) | 0.34811 (9) | 0.0261 (3) | |
| H1E | 0.2658 | 0.3233 | 0.3298 | 0.031* | |
| C2E | 0.19720 (12) | 0.28065 (4) | 0.37890 (9) | 0.0236 (3) | |
| C3E | 0.21231 (12) | 0.24610 (4) | 0.41326 (9) | 0.0221 (3) | |
| C4E | 0.32526 (13) | 0.23366 (4) | 0.40462 (10) | 0.0249 (3) | |
| C5E | 0.40861 (13) | 0.25255 (4) | 0.37280 (9) | 0.0265 (3) | |
| H5E | 0.4814 | 0.2430 | 0.3705 | 0.032* | |
| C6E | 0.38536 (13) | 0.28565 (4) | 0.34421 (9) | 0.0270 (3) | |
| N4A | 0.75790 (11) | 0.09105 (3) | 0.43652 (9) | 0.0303 (3) | |
| N5A | 0.69358 (11) | 0.14260 (3) | 0.39781 (8) | 0.0244 (3) | |
| N6A | 0.90342 (12) | 0.23186 (3) | 0.47233 (9) | 0.0253 (3) | |
| H6AA | 0.9724 (18) | 0.2348 (5) | 0.4655 (12) | 0.032 (5)* | |
| H6AB | 0.8939 (15) | 0.2239 (5) | 0.5237 (13) | 0.030 (5)* | |
| H6AC | 0.8659 (16) | 0.2535 (5) | 0.4655 (12) | 0.034 (5)* | |
| C7A | 0.66412 (14) | 0.09844 (4) | 0.47645 (10) | 0.0295 (3) | |
| H7A | 0.6323 | 0.0835 | 0.5146 | 0.035* | |
| C8A | 0.77269 (13) | 0.11818 (4) | 0.38983 (11) | 0.0276 (3) | |
| H8A | 0.8319 | 0.1203 | 0.3547 | 0.033* | |
| C9A | 0.62334 (13) | 0.13017 (4) | 0.45358 (10) | 0.0279 (3) | |
| H9A | 0.5595 | 0.1414 | 0.4723 | 0.033* | |
| C10A | 0.68255 (14) | 0.17569 (4) | 0.35409 (10) | 0.0284 (3) | |
| H10A | 0.6025 | 0.1801 | 0.3383 | 0.034* | |
| H10B | 0.7224 | 0.1742 | 0.3035 | 0.034* | |
| C11A | 0.72981 (13) | 0.20599 (4) | 0.40554 (11) | 0.0271 (3) | |
| H11A | 0.7011 | 0.2281 | 0.3812 | 0.033* | |
| H11B | 0.7038 | 0.2042 | 0.4612 | 0.033* | |
| C12A | 0.85708 (13) | 0.20646 (4) | 0.41082 (11) | 0.0277 (3) | |
| H12A | 0.8856 | 0.1830 | 0.4255 | 0.033* | |
| H12B | 0.8830 | 0.2125 | 0.3567 | 0.033* | |
| N4B | 0.73741 (12) | 0.39002 (3) | 0.09045 (10) | 0.0337 (3) | |
| N5B | 0.80676 (11) | 0.44049 (3) | 0.13254 (8) | 0.0250 (3) | |
| N6B | 0.61280 (11) | 0.53074 (3) | 0.04321 (9) | 0.0245 (3) | |
| H6BA | 0.6219 (15) | 0.5212 (5) | −0.0074 (12) | 0.029 (5)* | |
| H6BB | 0.5412 (18) | 0.5353 (5) | 0.0484 (12) | 0.040 (6)* | |
| H6BC | 0.6517 (15) | 0.5514 (5) | 0.0468 (11) | 0.029 (5)* | |
| C7B | 0.83655 (14) | 0.39557 (4) | 0.05633 (11) | 0.0305 (4) | |
| H7B | 0.8699 | 0.3800 | 0.0204 | 0.037* | |
| C8B | 0.72217 (14) | 0.41758 (4) | 0.13569 (11) | 0.0309 (4) | |
| H8B | 0.6592 | 0.4209 | 0.1668 | 0.037* | |
| C9B | 0.88023 (13) | 0.42658 (4) | 0.08124 (10) | 0.0280 (3) | |
| H9B | 0.9481 | 0.4367 | 0.0662 | 0.034* | |
| C10B | 0.81931 (14) | 0.47403 (4) | 0.17389 (10) | 0.0288 (3) | |
| H10C | 0.8990 | 0.4776 | 0.1924 | 0.035* | |
| H10D | 0.7757 | 0.4739 | 0.2228 | 0.035* | |
| C11B | 0.77981 (13) | 0.50414 (4) | 0.11863 (10) | 0.0265 (3) | |
| H11C | 0.8114 | 0.5261 | 0.1416 | 0.032* | |
| H11D | 0.8080 | 0.5008 | 0.0640 | 0.032* | |
| C12B | 0.65295 (13) | 0.50676 (4) | 0.11003 (10) | 0.0269 (3) | |
| H12C | 0.6209 | 0.4834 | 0.0989 | 0.032* | |
| H12D | 0.6260 | 0.5151 | 0.1621 | 0.032* | |
| N4C | 0.77635 (12) | 0.29279 (3) | 0.44507 (10) | 0.0333 (3) | |
| N5C | 0.71008 (11) | 0.34335 (3) | 0.40054 (9) | 0.0267 (3) | |
| N6C | 0.91077 (12) | 0.43386 (3) | 0.47087 (9) | 0.0238 (3) | |
| H6CA | 0.9797 (17) | 0.4372 (5) | 0.4660 (11) | 0.027 (5)* | |
| H6CB | 0.9011 (15) | 0.4268 (5) | 0.5215 (12) | 0.026 (5)* | |
| H6CC | 0.8729 (16) | 0.4547 (5) | 0.4643 (12) | 0.034 (5)* | |
| C7C | 0.68308 (15) | 0.30101 (4) | 0.48517 (11) | 0.0326 (4) | |
| H7C | 0.6523 | 0.2870 | 0.5255 | 0.039* | |
| C8C | 0.64165 (14) | 0.33207 (4) | 0.45851 (11) | 0.0310 (4) | |
| H8C | 0.5779 | 0.3437 | 0.4764 | 0.037* | |
| C9C | 0.78943 (14) | 0.31884 (4) | 0.39487 (11) | 0.0302 (4) | |
| H9C | 0.8478 | 0.3202 | 0.3589 | 0.036* | |
| C10C | 0.69767 (14) | 0.37533 (4) | 0.35294 (11) | 0.0300 (4) | |
| H10E | 0.6176 | 0.3788 | 0.3355 | 0.036* | |
| H10F | 0.7390 | 0.3730 | 0.3032 | 0.036* | |
| C11C | 0.74117 (13) | 0.40700 (4) | 0.40111 (11) | 0.0278 (3) | |
| H11E | 0.7123 | 0.4283 | 0.3733 | 0.033* | |
| H11F | 0.7129 | 0.4064 | 0.4562 | 0.033* | |
| C12C | 0.86828 (13) | 0.40808 (4) | 0.40902 (10) | 0.0273 (3) | |
| H12E | 0.8975 | 0.3848 | 0.4247 | 0.033* | |
| H12F | 0.8961 | 0.4141 | 0.3555 | 0.033* | |
| N4D | 0.22010 (12) | 0.50702 (3) | 0.56986 (9) | 0.0317 (3) | |
| N5D | 0.28930 (11) | 0.46050 (3) | 0.63391 (8) | 0.0258 (3) | |
| N6D | 0.11287 (12) | 0.36876 (3) | 0.55463 (9) | 0.0233 (3) | |
| H6DA | 0.0399 (17) | 0.3640 (5) | 0.5526 (11) | 0.029 (5)* | |
| H6DB | 0.1314 (16) | 0.3766 (5) | 0.5062 (13) | 0.035 (5)* | |
| H6DC | 0.1483 (15) | 0.3488 (5) | 0.5657 (11) | 0.028 (5)* | |
| C7D | 0.36432 (14) | 0.47005 (4) | 0.57872 (10) | 0.0298 (3) | |
| H7D | 0.4329 | 0.4589 | 0.5693 | 0.036* | |
| C8D | 0.20416 (13) | 0.48345 (4) | 0.62573 (10) | 0.0277 (3) | |
| H8D | 0.1403 | 0.4827 | 0.6567 | 0.033* | |
| C9D | 0.32091 (14) | 0.49861 (4) | 0.54037 (11) | 0.0318 (4) | |
| H9D | 0.3553 | 0.5111 | 0.4990 | 0.038* | |
| C10D | 0.29807 (14) | 0.43027 (4) | 0.68810 (10) | 0.0289 (3) | |
| H10G | 0.2483 | 0.4336 | 0.7330 | 0.035* | |
| H10H | 0.3758 | 0.4284 | 0.7126 | 0.035* | |
| C11D | 0.26634 (13) | 0.39670 (4) | 0.64306 (10) | 0.0271 (3) | |
| H11G | 0.3086 | 0.3950 | 0.5937 | 0.033* | |
| H11H | 0.2875 | 0.3767 | 0.6787 | 0.033* | |
| C12D | 0.14198 (13) | 0.39512 (4) | 0.61840 (10) | 0.0282 (3) | |
| H12G | 0.1013 | 0.3898 | 0.6672 | 0.034* | |
| H12H | 0.1167 | 0.4182 | 0.5979 | 0.034* | |
| N4E | 0.23642 (12) | 0.30890 (4) | 0.59484 (10) | 0.0341 (3) | |
| N5E | 0.30112 (11) | 0.26041 (3) | 0.65175 (8) | 0.0264 (3) | |
| N6E | 0.11830 (12) | 0.17068 (3) | 0.55652 (9) | 0.0244 (3) | |
| H6EA | 0.0441 (19) | 0.1656 (5) | 0.5537 (13) | 0.040 (6)* | |
| H6EB | 0.1349 (16) | 0.1796 (5) | 0.5102 (13) | 0.031 (5)* | |
| H6EC | 0.1545 (17) | 0.1495 (5) | 0.5656 (12) | 0.038 (5)* | |
| C7E | 0.34032 (15) | 0.30216 (4) | 0.56911 (11) | 0.0334 (4) | |
| H7E | 0.3782 | 0.3163 | 0.5326 | 0.040* | |
| C8E | 0.21567 (14) | 0.28319 (4) | 0.64442 (11) | 0.0308 (4) | |
| H8E | 0.1488 | 0.2810 | 0.6716 | 0.037* | |
| C9E | 0.38128 (14) | 0.27238 (4) | 0.60320 (11) | 0.0314 (4) | |
| H9E | 0.4514 | 0.2619 | 0.5952 | 0.038* | |
| C10E | 0.30688 (15) | 0.22809 (4) | 0.69875 (10) | 0.0309 (4) | |
| H10I | 0.2562 | 0.2298 | 0.7436 | 0.037* | |
| H10J | 0.3840 | 0.2249 | 0.7235 | 0.037* | |
| C11E | 0.27412 (14) | 0.19658 (4) | 0.64595 (11) | 0.0289 (3) | |
| H11I | 0.3144 | 0.1974 | 0.5955 | 0.035* | |
| H11J | 0.2970 | 0.1751 | 0.6759 | 0.035* | |
| C12E | 0.14910 (13) | 0.19551 (4) | 0.62346 (10) | 0.0285 (3) | |
| H12I | 0.1101 | 0.1889 | 0.6722 | 0.034* | |
| H12J | 0.1233 | 0.2190 | 0.6066 | 0.034* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1A | 0.0449 (8) | 0.0491 (9) | 0.0694 (10) | 0.0187 (7) | 0.0076 (7) | 0.0317 (8) |
| O2A | 0.0382 (7) | 0.0381 (7) | 0.0701 (10) | 0.0121 (6) | 0.0267 (7) | 0.0157 (7) |
| O3A | 0.0234 (6) | 0.0277 (6) | 0.0385 (7) | 0.0032 (4) | 0.0086 (5) | 0.0102 (5) |
| O4A | 0.0497 (8) | 0.0259 (7) | 0.0754 (10) | −0.0009 (6) | 0.0289 (7) | 0.0023 (6) |
| O5A | 0.0416 (8) | 0.0411 (8) | 0.1072 (14) | 0.0184 (7) | −0.0008 (8) | 0.0171 (9) |
| O6A | 0.0233 (6) | 0.0594 (9) | 0.0435 (7) | 0.0038 (6) | 0.0105 (5) | 0.0068 (6) |
| O7A | 0.0383 (7) | 0.0441 (8) | 0.0531 (8) | −0.0064 (6) | 0.0156 (6) | 0.0134 (6) |
| N1A | 0.0243 (7) | 0.0262 (7) | 0.0490 (9) | 0.0023 (6) | 0.0077 (6) | 0.0102 (6) |
| N2A | 0.0376 (8) | 0.0271 (7) | 0.0323 (8) | 0.0070 (6) | 0.0076 (6) | 0.0011 (6) |
| N3A | 0.0255 (7) | 0.0429 (9) | 0.0275 (7) | −0.0041 (6) | 0.0058 (6) | 0.0023 (6) |
| C1A | 0.0260 (8) | 0.0261 (8) | 0.0257 (8) | −0.0018 (6) | 0.0018 (6) | 0.0033 (6) |
| C2A | 0.0202 (7) | 0.0255 (8) | 0.0278 (8) | 0.0017 (6) | 0.0018 (6) | 0.0035 (6) |
| C3A | 0.0203 (7) | 0.0244 (8) | 0.0221 (7) | −0.0002 (6) | −0.0003 (6) | 0.0012 (6) |
| C4A | 0.0261 (8) | 0.0250 (8) | 0.0226 (7) | 0.0023 (6) | 0.0011 (6) | 0.0014 (6) |
| C5A | 0.0216 (8) | 0.0341 (9) | 0.0227 (8) | 0.0043 (6) | 0.0026 (6) | −0.0016 (6) |
| C6A | 0.0219 (8) | 0.0336 (9) | 0.0229 (8) | −0.0027 (6) | 0.0045 (6) | −0.0003 (6) |
| O1B | 0.0373 (7) | 0.0221 (6) | 0.0538 (8) | −0.0069 (5) | 0.0042 (6) | 0.0045 (5) |
| O2B | 0.0200 (6) | 0.0304 (6) | 0.0647 (9) | −0.0008 (5) | 0.0011 (5) | −0.0070 (6) |
| O3B | 0.0235 (5) | 0.0231 (5) | 0.0322 (6) | −0.0030 (4) | 0.0061 (4) | −0.0062 (4) |
| O4B | 0.0530 (8) | 0.0236 (6) | 0.0551 (8) | −0.0036 (6) | 0.0182 (7) | −0.0002 (6) |
| O5B | 0.0431 (8) | 0.0511 (9) | 0.0791 (11) | −0.0284 (7) | 0.0180 (8) | −0.0235 (8) |
| O6B | 0.0230 (6) | 0.0589 (8) | 0.0369 (7) | 0.0004 (6) | 0.0098 (5) | −0.0014 (6) |
| O7B | 0.0309 (6) | 0.0438 (8) | 0.0407 (7) | 0.0103 (5) | −0.0015 (5) | −0.0134 (6) |
| N1B | 0.0236 (7) | 0.0428 (8) | 0.0231 (7) | 0.0060 (6) | 0.0000 (5) | −0.0044 (6) |
| N2B | 0.0387 (8) | 0.0298 (8) | 0.0325 (8) | −0.0143 (6) | 0.0147 (6) | −0.0058 (6) |
| N3B | 0.0232 (7) | 0.0208 (6) | 0.0295 (7) | −0.0022 (5) | 0.0050 (5) | −0.0039 (5) |
| C1B | 0.0250 (8) | 0.0230 (7) | 0.0212 (7) | 0.0012 (6) | −0.0017 (6) | −0.0012 (6) |
| C2B | 0.0207 (7) | 0.0349 (9) | 0.0208 (7) | 0.0031 (6) | 0.0014 (6) | −0.0009 (6) |
| C3B | 0.0214 (7) | 0.0341 (9) | 0.0206 (7) | −0.0067 (6) | 0.0031 (6) | 0.0010 (6) |
| C4B | 0.0278 (8) | 0.0252 (8) | 0.0220 (7) | −0.0067 (6) | 0.0037 (6) | −0.0015 (6) |
| C5B | 0.0199 (7) | 0.0226 (7) | 0.0201 (7) | −0.0010 (6) | 0.0011 (5) | 0.0007 (6) |
| C6B | 0.0195 (7) | 0.0222 (7) | 0.0224 (7) | −0.0029 (6) | 0.0013 (6) | 0.0006 (6) |
| O1C | 0.0330 (7) | 0.0508 (8) | 0.0523 (8) | 0.0039 (6) | −0.0072 (6) | 0.0256 (7) |
| O2C | 0.0337 (7) | 0.0504 (8) | 0.0702 (10) | 0.0179 (6) | 0.0259 (7) | 0.0294 (7) |
| O3C | 0.0223 (5) | 0.0256 (6) | 0.0351 (6) | 0.0003 (4) | 0.0075 (5) | 0.0091 (5) |
| O4C | 0.0440 (7) | 0.0218 (6) | 0.0542 (8) | 0.0026 (5) | 0.0191 (6) | 0.0055 (5) |
| O5C | 0.0311 (7) | 0.0484 (8) | 0.0668 (10) | 0.0159 (6) | 0.0038 (6) | 0.0232 (7) |
| O6C | 0.0219 (6) | 0.0472 (7) | 0.0388 (7) | −0.0020 (5) | 0.0091 (5) | 0.0035 (6) |
| O7C | 0.0344 (7) | 0.0417 (7) | 0.0449 (7) | −0.0061 (5) | 0.0092 (6) | 0.0163 (6) |
| N1C | 0.0206 (7) | 0.0277 (7) | 0.0439 (8) | −0.0014 (5) | 0.0019 (6) | 0.0150 (6) |
| N2C | 0.0305 (8) | 0.0259 (7) | 0.0371 (8) | 0.0084 (6) | 0.0140 (6) | 0.0057 (6) |
| N3C | 0.0249 (7) | 0.0363 (8) | 0.0253 (7) | −0.0066 (6) | 0.0051 (5) | 0.0024 (6) |
| C1C | 0.0250 (8) | 0.0239 (7) | 0.0219 (7) | −0.0031 (6) | 0.0010 (6) | 0.0031 (6) |
| C2C | 0.0202 (7) | 0.0249 (8) | 0.0236 (7) | −0.0009 (6) | 0.0004 (6) | 0.0019 (6) |
| C3C | 0.0213 (7) | 0.0227 (7) | 0.0213 (7) | −0.0017 (6) | 0.0022 (6) | 0.0017 (6) |
| C4C | 0.0267 (8) | 0.0215 (7) | 0.0249 (8) | 0.0021 (6) | 0.0038 (6) | 0.0015 (6) |
| C5C | 0.0215 (7) | 0.0290 (8) | 0.0267 (8) | 0.0024 (6) | 0.0057 (6) | −0.0011 (6) |
| C6C | 0.0222 (8) | 0.0288 (8) | 0.0218 (7) | −0.0051 (6) | 0.0054 (6) | 0.0000 (6) |
| O1D | 0.0514 (9) | 0.0660 (11) | 0.1123 (15) | −0.0325 (8) | 0.0321 (10) | −0.0561 (11) |
| O2D | 0.0426 (8) | 0.0415 (8) | 0.0634 (10) | −0.0059 (6) | 0.0214 (7) | −0.0024 (7) |
| O3D | 0.0250 (6) | 0.0276 (6) | 0.0445 (7) | −0.0016 (5) | 0.0075 (5) | −0.0118 (5) |
| O4D | 0.0878 (13) | 0.0257 (7) | 0.1225 (16) | −0.0057 (8) | 0.0709 (12) | −0.0080 (8) |
| O5D | 0.038 (2) | 0.045 (2) | 0.141 (16) | −0.0225 (16) | 0.015 (4) | −0.031 (5) |
| O5DA | 0.045 (2) | 0.035 (2) | 0.075 (7) | −0.0157 (16) | 0.025 (3) | −0.008 (3) |
| O6D | 0.0262 (6) | 0.0556 (8) | 0.0445 (8) | −0.0018 (6) | 0.0111 (5) | −0.0065 (6) |
| O7D | 0.0333 (7) | 0.0399 (7) | 0.0446 (7) | 0.0085 (5) | 0.0031 (5) | −0.0130 (6) |
| N1D | 0.0243 (7) | 0.0248 (7) | 0.0523 (9) | −0.0015 (5) | 0.0087 (6) | −0.0080 (6) |
| N2D | 0.0384 (9) | 0.0290 (8) | 0.0369 (8) | −0.0069 (6) | 0.0062 (7) | −0.0043 (6) |
| N3D | 0.0245 (7) | 0.0423 (9) | 0.0260 (7) | 0.0038 (6) | 0.0007 (5) | −0.0049 (6) |
| C1D | 0.0253 (8) | 0.0242 (8) | 0.0280 (8) | 0.0015 (6) | −0.0001 (6) | −0.0052 (6) |
| C2D | 0.0211 (7) | 0.0244 (8) | 0.0283 (8) | −0.0016 (6) | 0.0015 (6) | −0.0022 (6) |
| C3D | 0.0211 (7) | 0.0251 (8) | 0.0246 (8) | 0.0003 (6) | −0.0004 (6) | −0.0040 (6) |
| C4D | 0.0271 (8) | 0.0264 (8) | 0.0238 (8) | −0.0030 (6) | 0.0000 (6) | −0.0025 (6) |
| C5D | 0.0220 (8) | 0.0343 (9) | 0.0252 (8) | −0.0041 (6) | 0.0025 (6) | −0.0005 (6) |
| C6D | 0.0227 (8) | 0.0334 (9) | 0.0237 (8) | 0.0028 (6) | 0.0020 (6) | −0.0027 (6) |
| O1E | 0.0381 (8) | 0.0269 (7) | 0.0914 (12) | 0.0065 (5) | 0.0036 (7) | 0.0034 (7) |
| O2E | 0.0224 (6) | 0.0378 (7) | 0.0552 (8) | −0.0012 (5) | −0.0018 (5) | 0.0052 (6) |
| O3E | 0.0225 (6) | 0.0262 (6) | 0.0341 (6) | −0.0002 (4) | 0.0068 (5) | 0.0058 (5) |
| O4E | 0.0464 (8) | 0.0228 (6) | 0.0692 (10) | −0.0008 (6) | 0.0221 (7) | −0.0001 (6) |
| O5E | 0.0317 (7) | 0.0446 (8) | 0.0922 (12) | 0.0163 (6) | 0.0089 (7) | 0.0199 (8) |
| O6E | 0.0254 (7) | 0.0687 (9) | 0.0358 (7) | −0.0100 (6) | 0.0100 (5) | 0.0017 (6) |
| O7E | 0.0402 (7) | 0.0501 (8) | 0.0457 (8) | −0.0185 (6) | −0.0040 (6) | 0.0210 (7) |
| N1E | 0.0236 (7) | 0.0273 (7) | 0.0315 (7) | −0.0001 (5) | 0.0020 (5) | 0.0073 (6) |
| N2E | 0.0322 (8) | 0.0280 (7) | 0.0449 (9) | 0.0089 (6) | 0.0164 (7) | 0.0009 (6) |
| N3E | 0.0282 (8) | 0.0489 (9) | 0.0234 (7) | −0.0141 (6) | −0.0014 (6) | 0.0073 (6) |
| C1E | 0.0277 (8) | 0.0277 (8) | 0.0222 (8) | −0.0055 (6) | −0.0019 (6) | 0.0045 (6) |
| C2E | 0.0203 (7) | 0.0263 (8) | 0.0240 (7) | −0.0010 (6) | 0.0003 (6) | 0.0008 (6) |
| C3E | 0.0219 (7) | 0.0227 (7) | 0.0219 (7) | −0.0018 (6) | 0.0022 (6) | −0.0019 (6) |
| C4E | 0.0247 (8) | 0.0240 (8) | 0.0266 (8) | 0.0001 (6) | 0.0060 (6) | −0.0009 (6) |
| C5E | 0.0208 (7) | 0.0341 (9) | 0.0251 (8) | −0.0004 (6) | 0.0051 (6) | −0.0037 (6) |
| C6E | 0.0239 (8) | 0.0365 (9) | 0.0206 (7) | −0.0096 (6) | 0.0017 (6) | 0.0015 (6) |
| N4A | 0.0278 (7) | 0.0204 (7) | 0.0421 (8) | 0.0044 (5) | −0.0006 (6) | 0.0008 (6) |
| N5A | 0.0233 (6) | 0.0186 (6) | 0.0311 (7) | 0.0019 (5) | 0.0003 (5) | 0.0001 (5) |
| N6A | 0.0192 (7) | 0.0209 (7) | 0.0364 (8) | 0.0005 (5) | 0.0060 (6) | 0.0041 (6) |
| C7A | 0.0299 (9) | 0.0250 (8) | 0.0333 (9) | 0.0003 (6) | 0.0009 (7) | 0.0036 (7) |
| C8A | 0.0236 (8) | 0.0211 (8) | 0.0384 (9) | 0.0033 (6) | 0.0039 (7) | −0.0018 (6) |
| C9A | 0.0251 (8) | 0.0263 (8) | 0.0325 (9) | 0.0042 (6) | 0.0041 (6) | −0.0012 (7) |
| C10A | 0.0293 (8) | 0.0214 (8) | 0.0337 (9) | 0.0026 (6) | −0.0021 (7) | 0.0042 (6) |
| C11A | 0.0240 (8) | 0.0193 (7) | 0.0382 (9) | 0.0031 (6) | 0.0035 (7) | 0.0011 (6) |
| C12A | 0.0243 (8) | 0.0225 (8) | 0.0372 (9) | 0.0026 (6) | 0.0080 (7) | −0.0010 (7) |
| N4B | 0.0290 (7) | 0.0199 (7) | 0.0516 (9) | −0.0043 (5) | −0.0009 (6) | 0.0004 (6) |
| N5B | 0.0234 (7) | 0.0219 (6) | 0.0294 (7) | −0.0031 (5) | −0.0003 (5) | 0.0008 (5) |
| N6B | 0.0204 (7) | 0.0190 (6) | 0.0347 (8) | −0.0027 (5) | 0.0064 (6) | −0.0037 (5) |
| C7B | 0.0298 (9) | 0.0260 (8) | 0.0352 (9) | 0.0004 (6) | −0.0003 (7) | −0.0016 (7) |
| C8B | 0.0253 (8) | 0.0244 (8) | 0.0435 (10) | −0.0037 (6) | 0.0058 (7) | 0.0037 (7) |
| C9B | 0.0238 (8) | 0.0267 (8) | 0.0338 (9) | −0.0033 (6) | 0.0027 (6) | −0.0003 (7) |
| C10B | 0.0299 (8) | 0.0268 (8) | 0.0294 (8) | −0.0037 (6) | −0.0009 (6) | −0.0042 (7) |
| C11B | 0.0252 (8) | 0.0204 (7) | 0.0343 (9) | −0.0044 (6) | 0.0048 (6) | −0.0029 (6) |
| C12B | 0.0248 (8) | 0.0238 (8) | 0.0329 (8) | −0.0039 (6) | 0.0078 (6) | 0.0002 (6) |
| N4C | 0.0319 (8) | 0.0211 (7) | 0.0462 (9) | 0.0051 (6) | −0.0016 (6) | −0.0013 (6) |
| N5C | 0.0248 (7) | 0.0193 (6) | 0.0353 (7) | 0.0032 (5) | −0.0013 (5) | −0.0022 (5) |
| N6C | 0.0186 (7) | 0.0226 (7) | 0.0304 (7) | 0.0003 (5) | 0.0034 (5) | 0.0042 (5) |
| C7C | 0.0349 (9) | 0.0269 (8) | 0.0358 (9) | 0.0014 (7) | 0.0013 (7) | 0.0009 (7) |
| C8C | 0.0284 (8) | 0.0283 (8) | 0.0363 (9) | 0.0061 (7) | 0.0026 (7) | −0.0032 (7) |
| C9C | 0.0264 (8) | 0.0210 (8) | 0.0433 (10) | 0.0021 (6) | 0.0020 (7) | −0.0036 (7) |
| C10C | 0.0299 (8) | 0.0232 (8) | 0.0358 (9) | 0.0019 (6) | −0.0047 (7) | 0.0017 (7) |
| C11C | 0.0226 (8) | 0.0206 (8) | 0.0399 (9) | 0.0035 (6) | 0.0002 (7) | −0.0001 (7) |
| C12C | 0.0248 (8) | 0.0250 (8) | 0.0325 (8) | 0.0034 (6) | 0.0047 (6) | −0.0012 (6) |
| N4D | 0.0304 (7) | 0.0217 (7) | 0.0421 (8) | 0.0027 (5) | −0.0029 (6) | −0.0012 (6) |
| N5D | 0.0236 (7) | 0.0222 (7) | 0.0309 (7) | 0.0034 (5) | −0.0024 (5) | −0.0023 (5) |
| N6D | 0.0218 (7) | 0.0198 (7) | 0.0290 (7) | 0.0016 (5) | 0.0049 (5) | 0.0026 (5) |
| C7D | 0.0245 (8) | 0.0292 (8) | 0.0355 (9) | 0.0028 (6) | 0.0018 (7) | −0.0033 (7) |
| C8D | 0.0231 (8) | 0.0232 (8) | 0.0369 (9) | 0.0029 (6) | 0.0020 (6) | −0.0048 (7) |
| C9D | 0.0315 (9) | 0.0294 (9) | 0.0342 (9) | −0.0021 (7) | 0.0017 (7) | −0.0004 (7) |
| C10D | 0.0286 (8) | 0.0279 (8) | 0.0294 (8) | 0.0044 (6) | −0.0036 (6) | 0.0012 (7) |
| C11D | 0.0258 (8) | 0.0236 (8) | 0.0316 (8) | 0.0056 (6) | −0.0009 (6) | 0.0014 (6) |
| C12D | 0.0258 (8) | 0.0275 (8) | 0.0317 (8) | 0.0036 (6) | 0.0051 (6) | −0.0048 (7) |
| N4E | 0.0329 (8) | 0.0224 (7) | 0.0462 (9) | 0.0037 (6) | −0.0022 (6) | 0.0007 (6) |
| N5E | 0.0263 (7) | 0.0217 (6) | 0.0307 (7) | 0.0037 (5) | −0.0018 (5) | −0.0007 (5) |
| N6E | 0.0218 (7) | 0.0200 (7) | 0.0319 (8) | 0.0015 (5) | 0.0056 (6) | 0.0022 (5) |
| C7E | 0.0328 (9) | 0.0277 (8) | 0.0395 (10) | 0.0000 (7) | 0.0014 (7) | 0.0027 (7) |
| C8E | 0.0286 (9) | 0.0238 (8) | 0.0400 (10) | 0.0042 (6) | 0.0027 (7) | −0.0040 (7) |
| C9E | 0.0251 (8) | 0.0305 (9) | 0.0386 (9) | 0.0033 (7) | 0.0025 (7) | −0.0006 (7) |
| C10E | 0.0345 (9) | 0.0277 (8) | 0.0295 (9) | 0.0030 (7) | −0.0049 (7) | 0.0038 (7) |
| C11E | 0.0286 (9) | 0.0232 (8) | 0.0346 (9) | 0.0043 (6) | −0.0008 (7) | 0.0027 (7) |
| C12E | 0.0280 (8) | 0.0273 (8) | 0.0307 (9) | 0.0032 (6) | 0.0056 (7) | −0.0030 (7) |
Geometric parameters (Å, º)
| O1A—N1A | 1.227 (2) | N6A—H6AC | 0.95 (2) |
| O2A—N1A | 1.224 (2) | N6A—C12A | 1.485 (2) |
| O3A—C3A | 1.2448 (18) | C7A—H7A | 0.9500 |
| O4A—N2A | 1.2268 (19) | C7A—C9A | 1.359 (2) |
| O5A—N2A | 1.2139 (19) | C8A—H8A | 0.9500 |
| O6A—N3A | 1.2322 (19) | C9A—H9A | 0.9500 |
| O7A—N3A | 1.223 (2) | C10A—H10A | 0.9900 |
| N1A—C2A | 1.459 (2) | C10A—H10B | 0.9900 |
| N2A—C4A | 1.449 (2) | C10A—C11A | 1.525 (2) |
| N3A—C6A | 1.449 (2) | C11A—H11A | 0.9900 |
| C1A—H1A | 0.9500 | C11A—H11B | 0.9900 |
| C1A—C2A | 1.373 (2) | C11A—C12A | 1.521 (2) |
| C1A—C6A | 1.396 (2) | C12A—H12A | 0.9900 |
| C2A—C3A | 1.452 (2) | C12A—H12B | 0.9900 |
| C3A—C4A | 1.453 (2) | N4B—C7B | 1.367 (2) |
| C4A—C5A | 1.376 (2) | N4B—C8B | 1.316 (2) |
| C5A—H5A | 0.9500 | N5B—C8B | 1.348 (2) |
| C5A—C6A | 1.376 (2) | N5B—C9B | 1.371 (2) |
| O1B—N3B | 1.2231 (17) | N5B—C10B | 1.462 (2) |
| O2B—N3B | 1.2270 (17) | N6B—H6BA | 0.92 (2) |
| O3B—C5B | 1.2451 (18) | N6B—H6BB | 0.89 (2) |
| O4B—N2B | 1.225 (2) | N6B—H6BC | 0.92 (2) |
| O5B—N2B | 1.2291 (19) | N6B—C12B | 1.487 (2) |
| O6B—N1B | 1.2295 (19) | C7B—H7B | 0.9500 |
| O7B—N1B | 1.2298 (19) | C7B—C9B | 1.355 (2) |
| N1B—C2B | 1.447 (2) | C8B—H8B | 0.9500 |
| N2B—C4B | 1.453 (2) | C9B—H9B | 0.9500 |
| N3B—C6B | 1.4567 (18) | C10B—H10C | 0.9900 |
| C1B—H1B | 0.9500 | C10B—H10D | 0.9900 |
| C1B—C2B | 1.391 (2) | C10B—C11B | 1.526 (2) |
| C1B—C6B | 1.371 (2) | C11B—H11C | 0.9900 |
| C2B—C3B | 1.380 (2) | C11B—H11D | 0.9900 |
| C3B—H3B | 0.9500 | C11B—C12B | 1.520 (2) |
| C3B—C4B | 1.371 (2) | C12B—H12C | 0.9900 |
| C4B—C5B | 1.452 (2) | C12B—H12D | 0.9900 |
| C5B—C6B | 1.451 (2) | N4C—C7C | 1.375 (2) |
| O1C—N1C | 1.2267 (18) | N4C—C9C | 1.315 (2) |
| O2C—N1C | 1.2206 (19) | N5C—C8C | 1.372 (2) |
| O3C—C3C | 1.2492 (18) | N5C—C9C | 1.348 (2) |
| O4C—N2C | 1.2238 (18) | N5C—C10C | 1.461 (2) |
| O5C—N2C | 1.235 (2) | N6C—H6CA | 0.85 (2) |
| O6C—N3C | 1.2329 (18) | N6C—H6CB | 0.89 (2) |
| O7C—N3C | 1.2273 (19) | N6C—H6CC | 0.92 (2) |
| N1C—C2C | 1.4523 (19) | N6C—C12C | 1.484 (2) |
| N2C—C4C | 1.446 (2) | C7C—H7C | 0.9500 |
| N3C—C6C | 1.4474 (19) | C7C—C8C | 1.356 (2) |
| C1C—H1C | 0.9500 | C8C—H8C | 0.9500 |
| C1C—C2C | 1.377 (2) | C9C—H9C | 0.9500 |
| C1C—C6C | 1.387 (2) | C10C—H10E | 0.9900 |
| C2C—C3C | 1.448 (2) | C10C—H10F | 0.9900 |
| C3C—C4C | 1.450 (2) | C10C—C11C | 1.525 (2) |
| C4C—C5C | 1.375 (2) | C11C—H11E | 0.9900 |
| C5C—H5C | 0.9500 | C11C—H11F | 0.9900 |
| C5C—C6C | 1.372 (2) | C11C—C12C | 1.520 (2) |
| O1D—N1D | 1.201 (2) | C12C—H12E | 0.9900 |
| O2D—N1D | 1.229 (2) | C12C—H12F | 0.9900 |
| O3D—C3D | 1.2469 (18) | N4D—C8D | 1.315 (2) |
| O4D—N2D | 1.198 (2) | N4D—C9D | 1.373 (2) |
| O5D—N2D | 1.172 (6) | N5D—C7D | 1.373 (2) |
| O5DA—N2D | 1.284 (6) | N5D—C8D | 1.3493 (19) |
| O6D—N3D | 1.2288 (19) | N5D—C10D | 1.465 (2) |
| O7D—N3D | 1.2276 (19) | N6D—H6DA | 0.89 (2) |
| N1D—C2D | 1.457 (2) | N6D—H6DB | 0.89 (2) |
| N2D—C4D | 1.451 (2) | N6D—H6DC | 0.89 (2) |
| N3D—C6D | 1.452 (2) | N6D—C12D | 1.482 (2) |
| C1D—H1D | 0.9500 | C7D—H7D | 0.9500 |
| C1D—C2D | 1.372 (2) | C7D—C9D | 1.353 (2) |
| C1D—C6D | 1.395 (2) | C8D—H8D | 0.9500 |
| C2D—C3D | 1.450 (2) | C9D—H9D | 0.9500 |
| C3D—C4D | 1.456 (2) | C10D—H10G | 0.9900 |
| C4D—C5D | 1.378 (2) | C10D—H10H | 0.9900 |
| C5D—H5D | 0.9500 | C10D—C11D | 1.524 (2) |
| C5D—C6D | 1.374 (2) | C11D—H11G | 0.9900 |
| O1E—N1E | 1.2253 (18) | C11D—H11H | 0.9900 |
| O2E—N1E | 1.2249 (18) | C11D—C12D | 1.517 (2) |
| O3E—C3E | 1.2472 (18) | C12D—H12G | 0.9900 |
| O4E—N2E | 1.2234 (19) | C12D—H12H | 0.9900 |
| O5E—N2E | 1.233 (2) | N4E—C7E | 1.369 (2) |
| O6E—N3E | 1.228 (2) | N4E—C8E | 1.317 (2) |
| O7E—N3E | 1.232 (2) | N5E—C8E | 1.347 (2) |
| N1E—C2E | 1.4546 (19) | N5E—C9E | 1.372 (2) |
| N2E—C4E | 1.448 (2) | N5E—C10E | 1.464 (2) |
| N3E—C6E | 1.448 (2) | N6E—H6EA | 0.91 (2) |
| C1E—H1E | 0.9500 | N6E—H6EB | 0.87 (2) |
| C1E—C2E | 1.373 (2) | N6E—H6EC | 0.93 (2) |
| C1E—C6E | 1.385 (2) | N6E—C12E | 1.483 (2) |
| C2E—C3E | 1.452 (2) | C7E—H7E | 0.9500 |
| C3E—C4E | 1.453 (2) | C7E—C9E | 1.353 (2) |
| C4E—C5E | 1.370 (2) | C8E—H8E | 0.9500 |
| C5E—H5E | 0.9500 | C9E—H9E | 0.9500 |
| C5E—C6E | 1.380 (2) | C10E—H10I | 0.9900 |
| N4A—C7A | 1.372 (2) | C10E—H10J | 0.9900 |
| N4A—C8A | 1.316 (2) | C10E—C11E | 1.526 (2) |
| N5A—C8A | 1.3485 (19) | C11E—H11I | 0.9900 |
| N5A—C9A | 1.374 (2) | C11E—H11J | 0.9900 |
| N5A—C10A | 1.4645 (19) | C11E—C12E | 1.517 (2) |
| N6A—H6AA | 0.85 (2) | C12E—H12I | 0.9900 |
| N6A—H6AB | 0.91 (2) | C12E—H12J | 0.9900 |
| O1A—N1A—C2A | 117.71 (14) | C11A—C10A—H10B | 109.2 |
| O2A—N1A—O1A | 123.60 (15) | C10A—C11A—H11A | 109.3 |
| O2A—N1A—C2A | 118.68 (14) | C10A—C11A—H11B | 109.3 |
| O4A—N2A—C4A | 119.55 (14) | H11A—C11A—H11B | 107.9 |
| O5A—N2A—O4A | 121.60 (15) | C12A—C11A—C10A | 111.75 (13) |
| O5A—N2A—C4A | 118.85 (15) | C12A—C11A—H11A | 109.3 |
| O6A—N3A—C6A | 117.84 (15) | C12A—C11A—H11B | 109.3 |
| O7A—N3A—O6A | 123.80 (14) | N6A—C12A—C11A | 111.71 (13) |
| O7A—N3A—C6A | 118.35 (14) | N6A—C12A—H12A | 109.3 |
| C2A—C1A—H1A | 120.6 | N6A—C12A—H12B | 109.3 |
| C2A—C1A—C6A | 118.77 (15) | C11A—C12A—H12A | 109.3 |
| C6A—C1A—H1A | 120.6 | C11A—C12A—H12B | 109.3 |
| C1A—C2A—N1A | 116.22 (14) | H12A—C12A—H12B | 107.9 |
| C1A—C2A—C3A | 124.90 (14) | C8B—N4B—C7B | 105.42 (14) |
| C3A—C2A—N1A | 118.87 (13) | C8B—N5B—C9B | 106.63 (13) |
| O3A—C3A—C2A | 124.92 (14) | C8B—N5B—C10B | 127.79 (14) |
| O3A—C3A—C4A | 124.07 (14) | C9B—N5B—C10B | 125.57 (13) |
| C2A—C3A—C4A | 111.01 (13) | H6BA—N6B—H6BB | 110.2 (17) |
| N2A—C4A—C3A | 119.08 (14) | H6BA—N6B—H6BC | 108.0 (16) |
| C5A—C4A—N2A | 116.44 (14) | H6BB—N6B—H6BC | 108.1 (17) |
| C5A—C4A—C3A | 124.48 (14) | C12B—N6B—H6BA | 111.4 (11) |
| C4A—C5A—H5A | 120.3 | C12B—N6B—H6BB | 108.3 (13) |
| C6A—C5A—C4A | 119.37 (14) | C12B—N6B—H6BC | 110.8 (12) |
| C6A—C5A—H5A | 120.3 | N4B—C7B—H7B | 125.0 |
| C1A—C6A—N3A | 118.97 (15) | C9B—C7B—N4B | 110.00 (15) |
| C5A—C6A—N3A | 120.01 (14) | C9B—C7B—H7B | 125.0 |
| C5A—C6A—C1A | 121.00 (14) | N4B—C8B—N5B | 111.75 (15) |
| O6B—N1B—O7B | 123.70 (14) | N4B—C8B—H8B | 124.1 |
| O6B—N1B—C2B | 118.19 (14) | N5B—C8B—H8B | 124.1 |
| O7B—N1B—C2B | 118.11 (14) | N5B—C9B—H9B | 126.9 |
| O4B—N2B—O5B | 123.03 (15) | C7B—C9B—N5B | 106.18 (14) |
| O4B—N2B—C4B | 119.13 (14) | C7B—C9B—H9B | 126.9 |
| O5B—N2B—C4B | 117.83 (15) | N5B—C10B—H10C | 109.2 |
| O1B—N3B—O2B | 123.41 (13) | N5B—C10B—H10D | 109.2 |
| O1B—N3B—C6B | 118.79 (13) | N5B—C10B—C11B | 112.23 (13) |
| O2B—N3B—C6B | 117.72 (13) | H10C—C10B—H10D | 107.9 |
| C2B—C1B—H1B | 120.6 | C11B—C10B—H10C | 109.2 |
| C6B—C1B—H1B | 120.6 | C11B—C10B—H10D | 109.2 |
| C6B—C1B—C2B | 118.72 (14) | C10B—C11B—H11C | 109.3 |
| C1B—C2B—N1B | 120.21 (15) | C10B—C11B—H11D | 109.3 |
| C3B—C2B—N1B | 118.68 (14) | H11C—C11B—H11D | 107.9 |
| C3B—C2B—C1B | 121.07 (14) | C12B—C11B—C10B | 111.74 (13) |
| C2B—C3B—H3B | 120.3 | C12B—C11B—H11C | 109.3 |
| C4B—C3B—C2B | 119.38 (14) | C12B—C11B—H11D | 109.3 |
| C4B—C3B—H3B | 120.3 | N6B—C12B—C11B | 112.16 (13) |
| C3B—C4B—N2B | 115.98 (14) | N6B—C12B—H12C | 109.2 |
| C3B—C4B—C5B | 124.57 (14) | N6B—C12B—H12D | 109.2 |
| C5B—C4B—N2B | 119.45 (14) | C11B—C12B—H12C | 109.2 |
| O3B—C5B—C4B | 124.26 (14) | C11B—C12B—H12D | 109.2 |
| O3B—C5B—C6B | 124.58 (13) | H12C—C12B—H12D | 107.9 |
| C6B—C5B—C4B | 110.99 (13) | C9C—N4C—C7C | 105.23 (14) |
| C1B—C6B—N3B | 116.43 (13) | C8C—N5C—C10C | 126.59 (14) |
| C1B—C6B—C5B | 125.00 (14) | C9C—N5C—C8C | 106.65 (14) |
| C5B—C6B—N3B | 118.57 (13) | C9C—N5C—C10C | 126.75 (15) |
| O1C—N1C—C2C | 118.16 (14) | H6CA—N6C—H6CB | 109.2 (17) |
| O2C—N1C—O1C | 123.32 (14) | H6CA—N6C—H6CC | 109.4 (17) |
| O2C—N1C—C2C | 118.50 (14) | H6CB—N6C—H6CC | 106.2 (16) |
| O4C—N2C—O5C | 123.11 (14) | C12C—N6C—H6CA | 109.0 (12) |
| O4C—N2C—C4C | 119.00 (14) | C12C—N6C—H6CB | 111.9 (12) |
| O5C—N2C—C4C | 117.88 (14) | C12C—N6C—H6CC | 111.1 (12) |
| O6C—N3C—C6C | 117.94 (14) | N4C—C7C—H7C | 125.1 |
| O7C—N3C—O6C | 123.59 (13) | C8C—C7C—N4C | 109.87 (16) |
| O7C—N3C—C6C | 118.46 (14) | C8C—C7C—H7C | 125.1 |
| C2C—C1C—H1C | 120.6 | N5C—C8C—H8C | 126.9 |
| C2C—C1C—C6C | 118.77 (14) | C7C—C8C—N5C | 106.29 (15) |
| C6C—C1C—H1C | 120.6 | C7C—C8C—H8C | 126.9 |
| C1C—C2C—N1C | 115.76 (13) | N4C—C9C—N5C | 111.97 (15) |
| C1C—C2C—C3C | 125.07 (14) | N4C—C9C—H9C | 124.0 |
| C3C—C2C—N1C | 119.16 (13) | N5C—C9C—H9C | 124.0 |
| O3C—C3C—C2C | 125.04 (14) | N5C—C10C—H10E | 109.1 |
| O3C—C3C—C4C | 124.28 (14) | N5C—C10C—H10F | 109.1 |
| C2C—C3C—C4C | 110.67 (13) | N5C—C10C—C11C | 112.30 (14) |
| N2C—C4C—C3C | 119.42 (13) | H10E—C10C—H10F | 107.9 |
| C5C—C4C—N2C | 115.46 (14) | C11C—C10C—H10E | 109.1 |
| C5C—C4C—C3C | 125.02 (14) | C11C—C10C—H10F | 109.1 |
| C4C—C5C—H5C | 120.4 | C10C—C11C—H11E | 109.3 |
| C6C—C5C—C4C | 119.16 (14) | C10C—C11C—H11F | 109.3 |
| C6C—C5C—H5C | 120.4 | H11E—C11C—H11F | 108.0 |
| C1C—C6C—N3C | 119.58 (14) | C12C—C11C—C10C | 111.63 (13) |
| C5C—C6C—N3C | 119.24 (14) | C12C—C11C—H11E | 109.3 |
| C5C—C6C—C1C | 121.18 (14) | C12C—C11C—H11F | 109.3 |
| O1D—N1D—O2D | 123.01 (16) | N6C—C12C—C11C | 111.62 (13) |
| O1D—N1D—C2D | 118.78 (15) | N6C—C12C—H12E | 109.3 |
| O2D—N1D—C2D | 118.13 (14) | N6C—C12C—H12F | 109.3 |
| O4D—N2D—O5DA | 121.1 (4) | C11C—C12C—H12E | 109.3 |
| O4D—N2D—C4D | 121.11 (15) | C11C—C12C—H12F | 109.3 |
| O5D—N2D—O4D | 116.2 (6) | H12E—C12C—H12F | 108.0 |
| O5D—N2D—C4D | 120.7 (4) | C8D—N4D—C9D | 105.07 (14) |
| O5DA—N2D—C4D | 114.8 (6) | C7D—N5D—C10D | 126.36 (13) |
| O6D—N3D—C6D | 117.99 (15) | C8D—N5D—C7D | 106.60 (14) |
| O7D—N3D—O6D | 123.75 (14) | C8D—N5D—C10D | 126.98 (14) |
| O7D—N3D—C6D | 118.26 (14) | H6DA—N6D—H6DB | 110.1 (17) |
| C2D—C1D—H1D | 120.7 | H6DA—N6D—H6DC | 106.3 (16) |
| C2D—C1D—C6D | 118.59 (15) | H6DB—N6D—H6DC | 109.1 (17) |
| C6D—C1D—H1D | 120.7 | C12D—N6D—H6DA | 110.3 (12) |
| C1D—C2D—N1D | 116.12 (14) | C12D—N6D—H6DB | 109.7 (13) |
| C1D—C2D—C3D | 125.24 (14) | C12D—N6D—H6DC | 111.3 (12) |
| C3D—C2D—N1D | 118.64 (13) | N5D—C7D—H7D | 127.0 |
| O3D—C3D—C2D | 123.99 (14) | C9D—C7D—N5D | 106.10 (14) |
| O3D—C3D—C4D | 124.81 (14) | C9D—C7D—H7D | 127.0 |
| C2D—C3D—C4D | 111.19 (13) | N4D—C8D—N5D | 111.99 (15) |
| N2D—C4D—C3D | 120.05 (14) | N4D—C8D—H8D | 124.0 |
| C5D—C4D—N2D | 116.02 (14) | N5D—C8D—H8D | 124.0 |
| C5D—C4D—C3D | 123.92 (15) | N4D—C9D—H9D | 124.9 |
| C4D—C5D—H5D | 120.0 | C7D—C9D—N4D | 110.24 (15) |
| C6D—C5D—C4D | 119.94 (15) | C7D—C9D—H9D | 124.9 |
| C6D—C5D—H5D | 120.0 | N5D—C10D—H10G | 109.2 |
| C1D—C6D—N3D | 119.68 (15) | N5D—C10D—H10H | 109.2 |
| C5D—C6D—N3D | 119.38 (14) | N5D—C10D—C11D | 112.06 (13) |
| C5D—C6D—C1D | 120.93 (15) | H10G—C10D—H10H | 107.9 |
| O1E—N1E—C2E | 118.81 (13) | C11D—C10D—H10G | 109.2 |
| O2E—N1E—O1E | 123.10 (14) | C11D—C10D—H10H | 109.2 |
| O2E—N1E—C2E | 118.02 (13) | C10D—C11D—H11G | 109.3 |
| O4E—N2E—O5E | 122.89 (15) | C10D—C11D—H11H | 109.3 |
| O4E—N2E—C4E | 119.37 (15) | H11G—C11D—H11H | 108.0 |
| O5E—N2E—C4E | 117.74 (15) | C12D—C11D—C10D | 111.64 (13) |
| O6E—N3E—O7E | 123.84 (15) | C12D—C11D—H11G | 109.3 |
| O6E—N3E—C6E | 118.14 (15) | C12D—C11D—H11H | 109.3 |
| O7E—N3E—C6E | 118.01 (15) | N6D—C12D—C11D | 113.07 (13) |
| C2E—C1E—H1E | 120.6 | N6D—C12D—H12G | 109.0 |
| C2E—C1E—C6E | 118.78 (15) | N6D—C12D—H12H | 109.0 |
| C6E—C1E—H1E | 120.6 | C11D—C12D—H12G | 109.0 |
| C1E—C2E—N1E | 116.32 (14) | C11D—C12D—H12H | 109.0 |
| C1E—C2E—C3E | 124.94 (14) | H12G—C12D—H12H | 107.8 |
| C3E—C2E—N1E | 118.74 (13) | C8E—N4E—C7E | 105.39 (14) |
| O3E—C3E—C2E | 124.73 (14) | C8E—N5E—C9E | 106.80 (14) |
| O3E—C3E—C4E | 124.29 (14) | C8E—N5E—C10E | 127.40 (15) |
| C2E—C3E—C4E | 110.81 (13) | C9E—N5E—C10E | 125.74 (14) |
| N2E—C4E—C3E | 119.12 (13) | H6EA—N6E—H6EB | 109.0 (18) |
| C5E—C4E—N2E | 115.91 (14) | H6EA—N6E—H6EC | 105.3 (17) |
| C5E—C4E—C3E | 124.92 (15) | H6EB—N6E—H6EC | 110.5 (17) |
| C4E—C5E—H5E | 120.5 | C12E—N6E—H6EA | 111.4 (13) |
| C4E—C5E—C6E | 119.08 (15) | C12E—N6E—H6EB | 109.5 (13) |
| C6E—C5E—H5E | 120.5 | C12E—N6E—H6EC | 111.0 (13) |
| C1E—C6E—N3E | 120.04 (15) | N4E—C7E—H7E | 124.9 |
| C5E—C6E—N3E | 118.65 (15) | C9E—C7E—N4E | 110.10 (16) |
| C5E—C6E—C1E | 121.31 (14) | C9E—C7E—H7E | 124.9 |
| C8A—N4A—C7A | 105.28 (13) | N4E—C8E—N5E | 111.62 (15) |
| C8A—N5A—C9A | 106.91 (13) | N4E—C8E—H8E | 124.2 |
| C8A—N5A—C10A | 126.75 (14) | N5E—C8E—H8E | 124.2 |
| C9A—N5A—C10A | 126.32 (13) | N5E—C9E—H9E | 127.0 |
| H6AA—N6A—H6AB | 110.8 (17) | C7E—C9E—N5E | 106.09 (15) |
| H6AA—N6A—H6AC | 109.0 (17) | C7E—C9E—H9E | 127.0 |
| H6AB—N6A—H6AC | 108.3 (16) | N5E—C10E—H10I | 109.2 |
| C12A—N6A—H6AA | 108.4 (13) | N5E—C10E—H10J | 109.2 |
| C12A—N6A—H6AB | 110.0 (12) | N5E—C10E—C11E | 112.11 (13) |
| C12A—N6A—H6AC | 110.3 (12) | H10I—C10E—H10J | 107.9 |
| N4A—C7A—H7A | 124.9 | C11E—C10E—H10I | 109.2 |
| C9A—C7A—N4A | 110.19 (15) | C11E—C10E—H10J | 109.2 |
| C9A—C7A—H7A | 124.9 | C10E—C11E—H11I | 109.3 |
| N4A—C8A—N5A | 111.82 (14) | C10E—C11E—H11J | 109.3 |
| N4A—C8A—H8A | 124.1 | H11I—C11E—H11J | 108.0 |
| N5A—C8A—H8A | 124.1 | C12E—C11E—C10E | 111.61 (13) |
| N5A—C9A—H9A | 127.1 | C12E—C11E—H11I | 109.3 |
| C7A—C9A—N5A | 105.80 (14) | C12E—C11E—H11J | 109.3 |
| C7A—C9A—H9A | 127.1 | N6E—C12E—C11E | 112.69 (13) |
| N5A—C10A—H10A | 109.2 | N6E—C12E—H12I | 109.1 |
| N5A—C10A—H10B | 109.2 | N6E—C12E—H12J | 109.1 |
| N5A—C10A—C11A | 112.22 (13) | C11E—C12E—H12I | 109.1 |
| H10A—C10A—H10B | 107.9 | C11E—C12E—H12J | 109.1 |
| C11A—C10A—H10A | 109.2 | H12I—C12E—H12J | 107.8 |
| O1A—N1A—C2A—C1A | 35.4 (2) | O7D—N3D—C6D—C5D | 179.93 (15) |
| O1A—N1A—C2A—C3A | −143.82 (17) | N1D—C2D—C3D—O3D | −0.7 (2) |
| O2A—N1A—C2A—C1A | −144.20 (17) | N1D—C2D—C3D—C4D | 178.26 (14) |
| O2A—N1A—C2A—C3A | 36.5 (2) | N2D—C4D—C5D—C6D | 174.80 (15) |
| O3A—C3A—C4A—N2A | 7.5 (2) | C1D—C2D—C3D—O3D | 179.78 (16) |
| O3A—C3A—C4A—C5A | −172.60 (15) | C1D—C2D—C3D—C4D | −1.3 (2) |
| O4A—N2A—C4A—C3A | 26.1 (2) | C2D—C1D—C6D—N3D | −176.99 (14) |
| O4A—N2A—C4A—C5A | −153.79 (16) | C2D—C1D—C6D—C5D | 3.4 (2) |
| O5A—N2A—C4A—C3A | −154.60 (17) | C2D—C3D—C4D—N2D | −174.13 (14) |
| O5A—N2A—C4A—C5A | 25.5 (2) | C2D—C3D—C4D—C5D | 4.4 (2) |
| O6A—N3A—C6A—C1A | −179.68 (14) | C3D—C4D—C5D—C6D | −3.8 (2) |
| O6A—N3A—C6A—C5A | −1.3 (2) | C4D—C5D—C6D—N3D | 179.98 (14) |
| O7A—N3A—C6A—C1A | 0.9 (2) | C4D—C5D—C6D—C1D | −0.4 (2) |
| O7A—N3A—C6A—C5A | 179.23 (15) | C6D—C1D—C2D—N1D | 178.02 (14) |
| N1A—C2A—C3A—O3A | −4.7 (2) | C6D—C1D—C2D—C3D | −2.4 (3) |
| N1A—C2A—C3A—C4A | 174.90 (14) | O1E—N1E—C2E—C1E | −33.6 (2) |
| N2A—C4A—C5A—C6A | 174.32 (14) | O1E—N1E—C2E—C3E | 145.62 (16) |
| C1A—C2A—C3A—O3A | 176.05 (16) | O2E—N1E—C2E—C1E | 143.49 (15) |
| C1A—C2A—C3A—C4A | −4.3 (2) | O2E—N1E—C2E—C3E | −37.3 (2) |
| C2A—C1A—C6A—N3A | −177.65 (14) | O3E—C3E—C4E—N2E | 5.2 (2) |
| C2A—C1A—C6A—C5A | 4.0 (2) | O3E—C3E—C4E—C5E | −171.92 (15) |
| C2A—C3A—C4A—N2A | −172.17 (14) | O4E—N2E—C4E—C3E | 34.7 (2) |
| C2A—C3A—C4A—C5A | 7.7 (2) | O4E—N2E—C4E—C5E | −147.92 (16) |
| C3A—C4A—C5A—C6A | −5.6 (2) | O5E—N2E—C4E—C3E | −144.93 (16) |
| C4A—C5A—C6A—N3A | −179.10 (14) | O5E—N2E—C4E—C5E | 32.4 (2) |
| C4A—C5A—C6A—C1A | −0.8 (2) | O6E—N3E—C6E—C1E | −172.73 (14) |
| C6A—C1A—C2A—N1A | 179.61 (14) | O6E—N3E—C6E—C5E | 6.7 (2) |
| C6A—C1A—C2A—C3A | −1.2 (2) | O7E—N3E—C6E—C1E | 8.1 (2) |
| O1B—N3B—C6B—C1B | −33.8 (2) | O7E—N3E—C6E—C5E | −172.40 (15) |
| O1B—N3B—C6B—C5B | 145.57 (15) | N1E—C2E—C3E—O3E | −8.5 (2) |
| O2B—N3B—C6B—C1B | 143.09 (15) | N1E—C2E—C3E—C4E | 176.08 (13) |
| O2B—N3B—C6B—C5B | −37.5 (2) | N2E—C4E—C5E—C6E | −179.10 (14) |
| O3B—C5B—C6B—N3B | −9.6 (2) | C1E—C2E—C3E—O3E | 170.70 (15) |
| O3B—C5B—C6B—C1B | 169.72 (15) | C1E—C2E—C3E—C4E | −4.8 (2) |
| O4B—N2B—C4B—C3B | −150.42 (15) | C2E—C1E—C6E—N3E | 177.47 (14) |
| O4B—N2B—C4B—C5B | 29.8 (2) | C2E—C1E—C6E—C5E | −2.0 (2) |
| O5B—N2B—C4B—C3B | 28.9 (2) | C2E—C3E—C4E—N2E | −179.32 (14) |
| O5B—N2B—C4B—C5B | −150.98 (15) | C2E—C3E—C4E—C5E | 3.6 (2) |
| O6B—N1B—C2B—C1B | −175.70 (14) | C3E—C4E—C5E—C6E | −1.9 (2) |
| O6B—N1B—C2B—C3B | 1.8 (2) | C4E—C5E—C6E—N3E | −178.58 (14) |
| O7B—N1B—C2B—C1B | 4.4 (2) | C4E—C5E—C6E—C1E | 0.9 (2) |
| O7B—N1B—C2B—C3B | −178.12 (14) | C6E—C1E—C2E—N1E | −176.60 (14) |
| N1B—C2B—C3B—C4B | −176.94 (14) | C6E—C1E—C2E—C3E | 4.2 (2) |
| N2B—C4B—C5B—O3B | 10.0 (2) | N4A—C7A—C9A—N5A | 0.25 (19) |
| N2B—C4B—C5B—C6B | −174.48 (14) | N5A—C10A—C11A—C12A | 75.73 (17) |
| C1B—C2B—C3B—C4B | 0.5 (2) | C7A—N4A—C8A—N5A | 0.13 (19) |
| C2B—C1B—C6B—N3B | −177.07 (13) | C8A—N4A—C7A—C9A | −0.24 (19) |
| C2B—C1B—C6B—C5B | 3.6 (2) | C8A—N5A—C9A—C7A | −0.17 (18) |
| C2B—C3B—C4B—N2B | 176.73 (14) | C8A—N5A—C10A—C11A | −99.79 (18) |
| C2B—C3B—C4B—C5B | −3.5 (2) | C9A—N5A—C8A—N4A | 0.02 (19) |
| C3B—C4B—C5B—O3B | −169.83 (15) | C9A—N5A—C10A—C11A | 81.85 (19) |
| C3B—C4B—C5B—C6B | 5.7 (2) | C10A—N5A—C8A—N4A | −178.60 (14) |
| C4B—C5B—C6B—N3B | 174.87 (13) | C10A—N5A—C9A—C7A | 178.46 (15) |
| C4B—C5B—C6B—C1B | −5.8 (2) | C10A—C11A—C12A—N6A | −169.81 (13) |
| C6B—C1B—C2B—N1B | 176.85 (13) | N4B—C7B—C9B—N5B | 0.42 (19) |
| C6B—C1B—C2B—C3B | −0.6 (2) | N5B—C10B—C11B—C12B | 77.05 (17) |
| O1C—N1C—C2C—C1C | −35.9 (2) | C7B—N4B—C8B—N5B | −0.2 (2) |
| O1C—N1C—C2C—C3C | 145.17 (15) | C8B—N4B—C7B—C9B | −0.1 (2) |
| O2C—N1C—C2C—C1C | 142.27 (16) | C8B—N5B—C9B—C7B | −0.53 (18) |
| O2C—N1C—C2C—C3C | −36.7 (2) | C8B—N5B—C10B—C11B | −96.84 (19) |
| O3C—C3C—C4C—N2C | −0.5 (2) | C9B—N5B—C8B—N4B | 0.48 (19) |
| O3C—C3C—C4C—C5C | 175.73 (15) | C9B—N5B—C10B—C11B | 82.11 (19) |
| O4C—N2C—C4C—C3C | −36.2 (2) | C10B—N5B—C8B—N4B | 179.59 (15) |
| O4C—N2C—C4C—C5C | 147.29 (15) | C10B—N5B—C9B—C7B | −179.66 (14) |
| O5C—N2C—C4C—C3C | 143.12 (16) | C10B—C11B—C12B—N6B | −167.69 (13) |
| O5C—N2C—C4C—C5C | −33.4 (2) | N4C—C7C—C8C—N5C | 0.2 (2) |
| O6C—N3C—C6C—C1C | 174.06 (14) | N5C—C10C—C11C—C12C | 74.86 (18) |
| O6C—N3C—C6C—C5C | −5.9 (2) | C7C—N4C—C9C—N5C | 0.14 (19) |
| O7C—N3C—C6C—C1C | −7.1 (2) | C8C—N5C—C9C—N4C | −0.01 (19) |
| O7C—N3C—C6C—C5C | 172.89 (15) | C8C—N5C—C10C—C11C | 79.6 (2) |
| N1C—C2C—C3C—O3C | 0.4 (2) | C9C—N4C—C7C—C8C | −0.2 (2) |
| N1C—C2C—C3C—C4C | 179.80 (13) | C9C—N5C—C8C—C7C | −0.12 (19) |
| N2C—C4C—C5C—C6C | 179.99 (14) | C9C—N5C—C10C—C11C | −101.93 (19) |
| C1C—C2C—C3C—O3C | −178.42 (15) | C10C—N5C—C8C—C7C | 178.57 (15) |
| C1C—C2C—C3C—C4C | 1.0 (2) | C10C—N5C—C9C—N4C | −178.70 (15) |
| C2C—C1C—C6C—N3C | 178.18 (14) | C10C—C11C—C12C—N6C | −166.96 (13) |
| C2C—C1C—C6C—C5C | −1.8 (2) | N5D—C7D—C9D—N4D | −0.43 (19) |
| C2C—C3C—C4C—N2C | −179.86 (14) | N5D—C10D—C11D—C12D | −70.02 (18) |
| C2C—C3C—C4C—C5C | −3.7 (2) | C7D—N5D—C8D—N4D | −0.51 (19) |
| C3C—C4C—C5C—C6C | 3.7 (2) | C7D—N5D—C10D—C11D | −77.33 (19) |
| C4C—C5C—C6C—N3C | 179.33 (14) | C8D—N4D—C9D—C7D | 0.13 (19) |
| C4C—C5C—C6C—C1C | −0.7 (2) | C8D—N5D—C7D—C9D | 0.56 (18) |
| C6C—C1C—C2C—N1C | −177.26 (14) | C8D—N5D—C10D—C11D | 99.47 (18) |
| C6C—C1C—C2C—C3C | 1.6 (2) | C9D—N4D—C8D—N5D | 0.24 (19) |
| O1D—N1D—C2D—C1D | 34.2 (2) | C10D—N5D—C7D—C9D | 177.90 (15) |
| O1D—N1D—C2D—C3D | −145.42 (19) | C10D—N5D—C8D—N4D | −177.83 (14) |
| O2D—N1D—C2D—C1D | −142.63 (16) | C10D—C11D—C12D—N6D | 162.89 (13) |
| O2D—N1D—C2D—C3D | 37.8 (2) | N4E—C7E—C9E—N5E | −0.4 (2) |
| O3D—C3D—C4D—N2D | 4.8 (2) | N5E—C10E—C11E—C12E | −73.02 (18) |
| O3D—C3D—C4D—C5D | −176.62 (16) | C7E—N4E—C8E—N5E | 0.1 (2) |
| O4D—N2D—C4D—C3D | 5.9 (3) | C8E—N4E—C7E—C9E | 0.2 (2) |
| O4D—N2D—C4D—C5D | −172.77 (19) | C8E—N5E—C9E—C7E | 0.41 (19) |
| O5D—N2D—C4D—C3D | −158 (2) | C8E—N5E—C10E—C11E | 95.96 (19) |
| O5D—N2D—C4D—C5D | 24 (2) | C9E—N5E—C8E—N4E | −0.3 (2) |
| O5DA—N2D—C4D—C3D | 166.4 (11) | C9E—N5E—C10E—C11E | −80.7 (2) |
| O5DA—N2D—C4D—C5D | −12.3 (11) | C10E—N5E—C8E—N4E | −177.52 (15) |
| O6D—N3D—C6D—C1D | 179.42 (15) | C10E—N5E—C9E—C7E | 177.67 (15) |
| O6D—N3D—C6D—C5D | −0.9 (2) | C10E—C11E—C12E—N6E | 165.79 (14) |
| O7D—N3D—C6D—C1D | 0.3 (2) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N6A—H6AA···O3Ei | 0.85 (2) | 2.06 (2) | 2.8943 (18) | 167.5 (18) |
| N6A—H6AB···O3Dii | 0.91 (2) | 2.08 (2) | 2.8671 (18) | 143.1 (16) |
| N6A—H6AB···O4Dii | 0.91 (2) | 2.25 (2) | 2.961 (2) | 134.6 (15) |
| N6A—H6AC···N4C | 0.95 (2) | 1.87 (2) | 2.8157 (19) | 172.5 (18) |
| N6B—H6BA···O3Biii | 0.92 (2) | 2.117 (19) | 2.8728 (17) | 138.5 (15) |
| N6B—H6BA···O4Biii | 0.92 (2) | 2.268 (19) | 3.006 (2) | 136.6 (15) |
| N6B—H6BB···O3B | 0.89 (2) | 2.00 (2) | 2.8502 (17) | 161.0 (19) |
| N6B—H6BC···N4Aiv | 0.92 (2) | 1.88 (2) | 2.7988 (19) | 173.4 (17) |
| N6C—H6CA···O2Cv | 0.85 (2) | 2.305 (18) | 2.8334 (19) | 120.9 (15) |
| N6C—H6CA···O3Cv | 0.85 (2) | 2.10 (2) | 2.8944 (18) | 155.4 (17) |
| N6C—H6CB···O3Avi | 0.89 (2) | 2.174 (19) | 2.9145 (18) | 140.2 (16) |
| N6C—H6CB···O4Avi | 0.89 (2) | 2.270 (19) | 2.986 (2) | 137.3 (15) |
| N6C—H6CC···N4Dvi | 0.92 (2) | 1.91 (2) | 2.8179 (19) | 167.6 (17) |
| N6D—H6DA···O2Avii | 0.89 (2) | 2.361 (19) | 2.9631 (19) | 124.9 (15) |
| N6D—H6DA···O3Avii | 0.89 (2) | 2.06 (2) | 2.8340 (17) | 145.0 (16) |
| N6D—H6DB···O3Cvi | 0.89 (2) | 2.16 (2) | 2.9171 (17) | 142.0 (17) |
| N6D—H6DB···O4Cvi | 0.89 (2) | 2.27 (2) | 2.9323 (19) | 130.5 (16) |
| N6D—H6DC···N4E | 0.89 (2) | 1.91 (2) | 2.7932 (19) | 174.0 (17) |
| N6E—H6EA···O2Dviii | 0.91 (2) | 2.32 (2) | 2.973 (2) | 128.7 (17) |
| N6E—H6EA···O3Dviii | 0.91 (2) | 2.06 (2) | 2.8527 (18) | 145.4 (18) |
| N6E—H6EB···O3E | 0.87 (2) | 2.19 (2) | 2.9056 (18) | 139.6 (17) |
| N6E—H6EB···O4E | 0.87 (2) | 2.33 (2) | 3.010 (2) | 135.3 (16) |
| N6E—H6EC···N4Bviii | 0.93 (2) | 1.85 (2) | 2.7750 (19) | 174.0 (19) |
| C8A—H8A···O2Bix | 0.95 | 2.46 | 3.0887 (19) | 123 |
| C9A—H9A···O5E | 0.95 | 2.43 | 3.243 (2) | 144 |
| C12A—H12B···O7Aix | 0.99 | 2.46 | 3.313 (2) | 145 |
| C9B—H9B···O5Bi | 0.95 | 2.37 | 3.224 (2) | 150 |
| C12B—H12D···O7Cvi | 0.99 | 2.47 | 3.335 (2) | 145 |
| C8C—H8C···O5Cvi | 0.95 | 2.33 | 3.194 (2) | 152 |
| C9C—H9C···O2Ei | 0.95 | 2.49 | 3.105 (2) | 123 |
| C7D—H7D···O5Avi | 0.95 | 2.35 | 3.233 (2) | 155 |
| C11D—H11H···O6Avi | 0.99 | 2.55 | 3.421 (2) | 147 |
| C12D—H12G···O6Bvii | 0.99 | 2.43 | 3.238 (2) | 139 |
| C9E—H9E···O5Dii | 0.95 | 2.27 | 3.162 (6) | 156 |
| C9E—H9E···O5DAii | 0.95 | 2.56 | 3.357 (17) | 141 |
| C12E—H12I···O6Eviii | 0.99 | 2.34 | 3.186 (2) | 142 |
Symmetry codes: (i) x+1, y, z; (ii) x+1/2, −y+1/2, z+1/2; (iii) −x+1, −y+1, −z; (iv) −x+3/2, y+1/2, −z+1/2; (v) −x+2, −y+1, −z+1; (vi) −x+1, −y+1, −z+1; (vii) −x, −y+1, −z+1; (viii) x−1/2, −y+1/2, z+1/2; (ix) −x+3/2, y−1/2, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SJ5352).
References
- Agilent (2012). CrysAlis PRO and CrysAlis RED Agilent Technologies, Yarnton, England.
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans 2, pp. S1–19.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Du, M. & Zhao, X.-J. (2003). Acta Cryst. E59, o1898–o1900.
- Dutkiewicz, G., Samshuddin, S., Narayana, B., Yathirajan, H. S. & Kubicki, M. (2011). Acta Cryst. E67, o235. [DOI] [PMC free article] [PubMed]
- In, Y., Nagata, H., Doi, M., Ishida, T. & Wakahara, A. (1997). Acta Cryst. C53, 367–369.
- Jin, C. M., Ye, C., Piekarski, C., Twamley, B. & Shreeve, J. M. (2005). Eur. J. Inorg. Chem. pp. 3760–3767.
- Krezel, I. (1998). Farmaco, 53, 342–345. [DOI] [PubMed]
- Lombardino, J. G. & Wiseman, E. H. (1974). J. Med. Chem. 17, 1182–1188. [DOI] [PubMed]
- MacDonald, J., Yigit, M. V. & Mychajlonka, K. (2005). Cryst. Growth Des. 5, 2248–2255.
- Maier, T., Schmierer, R., Bauer, K., Bieringer, H., Buerstell, H. & Sachse, B. (1989a). US Patent 4 820 335.
- Maier, T., Schmierer, R., Bauer, K., Bieringer, H., Buerstell, H. & Sachse, B. (1989b). Chem. Abstr. 111, 19494.
- Nardelli, M., Pelizzi, G., Vitali, F., Bordi, F., Plazzi, P. V. & Vitali, T. (1987). Acta Cryst. C43, 507–514.
- Palatinus, L. & Chapuis, G. (2007). J. Appl. Cryst. 40, 786–790.
- Pi, M., Liu, X.-L., Xu, J.-J. & Jin, C.-M. (2009). Acta Cryst. E65, o2386. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Soriano-García, M., Schatz-Levine, M., Toscano, R. A. & Villena Iribe, R. (1990). Acta Cryst. C46, 1556–1558.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S1600536813025646/sj5352sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813025646/sj5352Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813025646/sj5352Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report