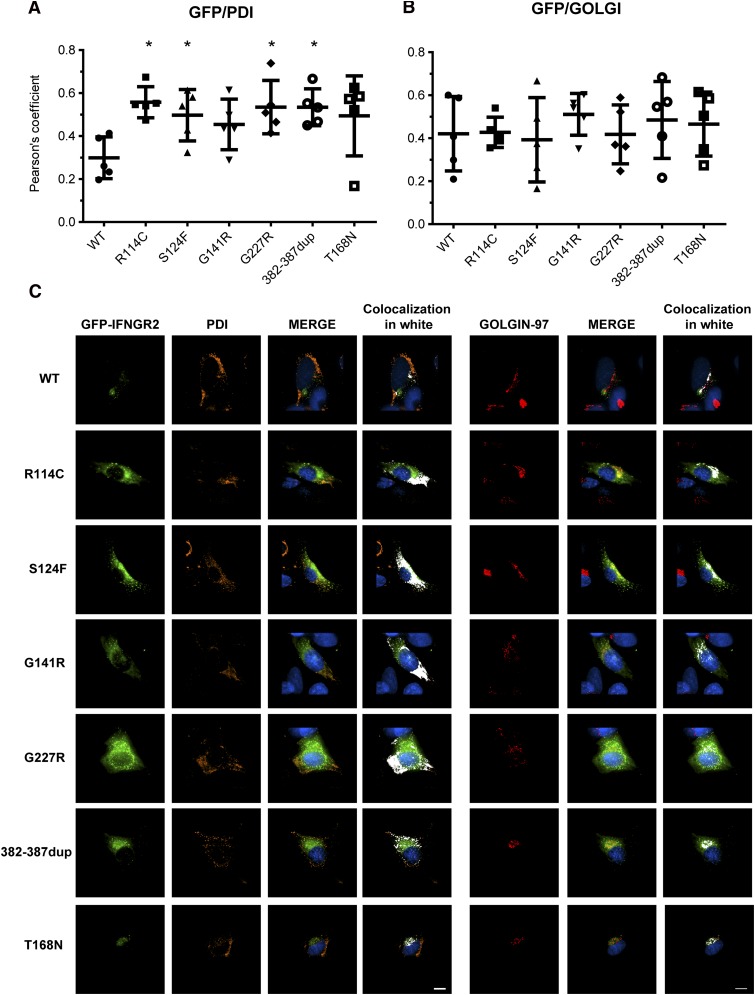
Figure 6.
Subcellular localization of mutant IFN-γR2 proteins. SV40 fibroblasts from the IFN-γR2–deficient patient (278delAG/278delAG) were transiently transfected for 48 hours with WT, S124F, R114C, G141R, G227R, 382-387dup, and T168N IFNGR2-tagged EGFP constructs. Cells were fixed and permeabilized, and intracellular double staining with PDI (orange) and golgin-97 (red) was performed. Images were acquired with DeltaVision Image Restoration Microscope with the 60× objective. Colocalization of GFP with (A) PDI and (B) golgin-97 was measured as Pearson’s coefficients in dataset volumes. The values shown are the mean ± standard deviation of 5 cells analyzed. Differences between WT and each mutant were analyzed using the Student t test. P < .05 was considered significant and is indicated by an asterisk. (C) Colocalization of WT and mutant proteins with PDI or golgin-97 were visualized by merging the images, and then a colocalization channel was created using IMARIS software. Colocalized pixels are show in white. Nuclei were stained with 4,6 diamidino-2-phenylindole (blue). Scale bars, 5 μm.