85.1 Introduction
Null mutations in the visual system homeobox 2 (Vsx2; a.k.a. Chx10) gene result in micro-ophthalmia and failed retinal development in man, mouse, and zebrafish (Burmeister et al. 1996; Barabino et al. 1997; Ferda Percin et al. 2000).Vsx2orJ/orJ (Chx10or, ocular retardation) mutant mice exhibit a 19-fold decrease in the number of retinal cells, optic nerve aplasia, dramatic (if not complete) loss of bipolar interneurons, and the retinas lack a normal layered structure (Burmeister et al. 1996). Previous studies have shown that there are fewer proliferating cells in the retinas of Vsx2orJ/orJmice during embryonic development, and that overproduction of retinal pigmented epithelium – in contrast to neural epithelium — is likely to be a major cause of the severe phenotypes observed in Vsx2 mutant retinas (Burmeister et al. 1996; Rowan et al. 2004). These observations make the Vsx2 mutant mouse a good model for understanding the onset, progression, and molecular etiology of development retinal dystrophies in man.
Growth and differentiation factor 11 (GDF11), a member of the TGF-β super-family of signaling molecules, has been shown to be an autocrine negative regulator of neurogenesis, as well as an important regulator of stem/progenitor cell fate, in sensory epithelia (Wu et al. 2003; Kim et al. 2005; Lander et al. 2009). In the olfactory epithelium (OE), Gdf11 negatively regulates neuronal cell number by causing cell-cycle arrest and/or differentiation of the progenitor cells that give rise to olfactory receptor neurons (ORNs), and as a result, the OE of Gdf11−/− mice show an increased number of both neuronal progenitors and ORNs, and is thicker than that of wildtype mice (Wu et al. 2003; Lander et al. 2009). In the retina, absence of Gdf11 results in an increase in the number of retinal ganglion cells (RGCs) (Fig. 85.1a), the earliest cell type to differentiate during retinal neurogenesis; however, this increase is at the expense of later-born cell types such as rod photoreceptors and amacrine cells (Kim et al. 2005). Multiple lines of evidence indicate that this effect is due to a change in the fate of retinal stem/progenitor cells, and is a consequence of prolonged expression of Math5 (Atoh7, MGI), a transcription factor that confers competence to form RGCs, in Gdf11−/− retina (Fig. 85.1b). GDF11 activity is required to downregulate expression of Math5, and in its absence, RGCs are produced for an extended period of time and accumulate in an aberrantly thick RGC layer (Fig. 85.1) and (Kim et al. 2005; Harada et al. 2007).
Fig. 85.1.
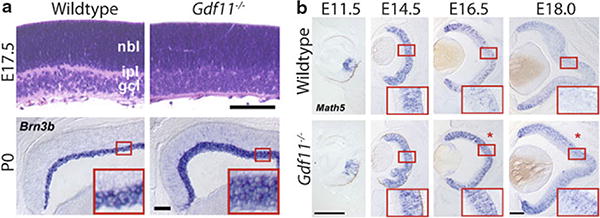
GDF11 is a negative regulator of retinal neurogenesis. (a) H&E stain at E17.5 and Brn3b ISH at P0 show an increase in RGC, but no change in overall thickness of Gdf11−/− retinas. (b) ISH showing prolonged expression of Math5 in Gdf11−/− retinas. Asterisks indicate reproducible Math5 upregulation in Gdf11−/− retina from wildtype controls. Scale bars: 100 μm (a), 200 μmm (b). Adapted from (Kim et al. 2005)
GDF11's role in modulating the activity of transcription factors that specify development of sensory epithelia and the cells within them has other consequences as well. For example, mice that lack the winged helix transcription factor FoxG1 lack an OE and have greatly reduced cerebral hemispheres (Xuan et al. 1995). We have found that the ability of FoxG1 to drive OE neurogenesis is due to its function as a negative regulator of GDF11 activity: Reduction of Gdf11 levels rescues, to a significant extent, both the early developmental loss of neuronal progenitor cells in Foxg1−/− OE, and the defects in nasal cavity and olfactory turbinate structure development seen in these mutants (Kawauchi et al. 2009). These effects are gene dose-dependent, with loss of even one allele of Gdf11 restoring full histogenesis of the OE neuroepithelium in those regions of the nasal cavity that now develop (Kawauchi et al. 2009).
Given these diverse yet related developmental functions of GDF11, we speculated that reducing GDF11 levels might promote restoration of retinal development in the micro-ophthalmic Vsx2orJ/orJ mouse. To test this idea, we used genetic manipulations to alter Gdf11 levels in the retinas of Vsx2orJ/orJ mouse, and examined retinal phenotypes at various developmental ages.
85.2 Materials and Methods
85.2.1 Animals
Gdf11+/− mice (Gdf11tm2/+) (Wu et al. 2003) maintained on a C57bl/6 background were bred with Vsx2orJ/orJ mice to generate Gdf11+/−;Vsx2orJ/+ mice; these mice were intercrossed, or were bred with mice heterozygous for one gene only, to obtain animals of the various genotypes studied. Day of vaginal plug discovery was designated embryonic day (E) 0.5. The Institutional Animal Care and Use Committee of the University of California, Irvine, approved all protocols for animal use.
85.2.2 Histology and In Situ Hybridization
Tissues were fixed in 4% paraformaldehyde, cryoprotected, embedded, and cryo-sectioned at 20μm as described (Murray et al. 2003). For in situ hybridization (ISH), cRNA probes for Brn3b (POU4F2), Crx, Foxn4, Math5 (Atoh7), and Neurod1 were synthesized and hybridized as described (Kim et al. 2005). Images were taken using a Zeiss Axiophot microscope equipped with AxioVision Software (Carl Zeiss, Thornwood, NY, USA).
85.3 Results
In a previous study, we noted that inactivation of one or two alleles of Gdf11 progressively restores development of OE in Foxg1−/− mice (Kawauchi et al. 2009). We performed a similar analysis by examining stained sections of retinas from Gdf11−/−;Vsx2orJ/orJ, Vsx2orJ/orJ and control (Vsx2orJ/+) mice at various developmental stages, and measuring thickness of central retina in each case. Reduction of Gdf11 levels rescues retinal thickness as early as day 14.5 of gestation (Table 85.1): The retina is about 20% thicker in Gdf11−/−;Vsx2orJ/orJ retinas than in Vsx2orJ/orJ retinas (although thickness is not restored to full control levels). Epithelial thicknesses and cell lamination were also noticeably rescued in Gdf11+/−;Vsx2orJ/orJ retinas, implying a dose-dependence of this effect; this was most readily observed at later ages, as shown in Fig. 85.2.
Table 85.1. Rescued expression of developmental genes in Vsx2orJ/orJ retinas.
| Gene | Gene Function | Age | Control | Vsx2orJ/orJ | Gdf11−/−;Vsx2orJ/orJ |
|---|---|---|---|---|---|
| Brn3b | RGC development (Erkman et al. 1996) | E14.5 | + + + + + | +/− | + + + |
| Neurod1 | Amacrine development (Harada et al. 2007) | E14.5 | + + + + + | +/− | + + |
| Crx | Photoreceptor development (Furukawa et al. 1999) | E14.5 | + + + + + | +/− | + |
| Foxn4 | Neuronal retina (Kelly et al. 2007) | E14.5 | + + + + + | − | + + + |
| Math5 | Competence to make RGCs (Harada et al. 2007) | E14.5 | + + + + + | + | + + + + |
| Thickness (increase relative to Vsx2orJ/orJ) | E14.5 | 95% | (0%) | 20% | |
Gene expression levels were scored from ISH and based on intensity and relative area of expression. “−” indicates no expression; “+/−” indicates low expression (<5 cells expressing); “+” indicates that expression was clearly observed. The strongest signal intensity with greatest expansion for each gene was scored as +++++. The scores of the various genes are compared to control retinas. Thickness of central retina was measured at E14.5
Fig. 85.2.
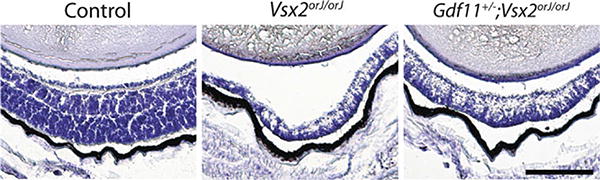
Partial rescue of retinal lamination and thickness in Vsx2orJ/orJ retina by inactivation of one allele of Gdf11 at P7. Scale bar: 200 μm
Expression of genes important for retinal development and neural cell differentiation show drastic reductions in their expression in Vsx2orJ/orJ retinas (Rowan et al. 2004). We performed ISH to examine expression of Brn3b, Neurod1, Crx, Foxn4, and Math5 in the retinas of Vsx2 mutants, and in these mice when Gdf11 levels were reduced. Absence of Gdf11 resulted in increased expression of these genes (Table 85.1). For example, Brn3b is expressed in the presumptive ganglion cell layer (GCL) at E14.5 in control retinas. In contrast, Vsx2orJ/orJ retinas exhibit a severe reduction in Brn3b expression, with only a few scattered cells expressing Brn3b. In Gdf11−/−;Vsx2orJ/orJ retinas, Brn3b expression is increased in intensity, expanded in area, and is localized to a clearly-demarcated developing GCL. Similarly, Foxn4 expression, normally expressed in stem/progenitor cells throughout the NBL, is absent in Vsx2orJ/orJ retinas. Rescued retinas show an increase in ISH intensity and expanded Foxn4 expression domain, restricted primarily to a demarcated NBL. Other retinal development genes show similar rescue of expression when Gdf11 levels are reduced (Table 85.1).
85.4 Discussion
In the present study, we show that Vsx2 mutant retinas can be rescued by reducing Gdf11 activity and that this rescue is gene dosage dependent. Expression analysis shows that genes required for proper retina development are minimally expressed or absent in the central region of Vsx2orJ/orJ retina. With loss of Gdf11 (Gdf11−/−;Vsx2orJ/orJ retinas), expression of these genes is expanded, and the retinal neuroepithelium displays a more-normal lamination pattern and is increased in thickness (Fig. 85.3). These observations indicate that cells within the Vsx2 mutant retina retain the potential to produce differentiated neuronal cell types, and imply that this process is negatively regulated by the action of GDF11. Further support for this idea comes from the observation that reducing levels of follistatin (a high-affinity GDF11 antagonist expressed within neural retina; cf. (Kim et al. 2005)) in Vsx2 mutants exacerbates the mutant phenotype (data not shown).
Fig. 85.3.
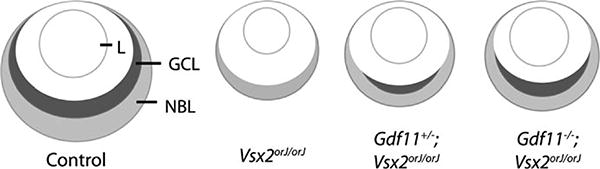
Restoration of retinal development in Vsx2orJ/orJ mice by reduction of Gdf11 activity. Schematic model showing a section through a control eye at E14.5, with the developing ganglion cell layer (GCL) and neuroblastic layer (NBL) indicated. Vsx2orJ/orJ eyes are smaller than controls, and have thinner retinas with no obvious lamination. Gdf11+/−;Vsx2orJ/orJ eyes are larger and their retinas are thicker than Vsx2orJ/orJ retinas and show some lamination, including partial development of the GCL. Gdf11−/−;Vsx2orJ/orJ eyes and retinas are larger/thicker still, with clearly-visible GCL and NBL. L, lens
Interestingly, recent preliminary studies suggest that not only is developmental gene expression rescued by reducing Gdf11 levels in Vsx2orJ/orJ retinas; optic nerve development may be rescued as well: DiI tracing of RGC axons in perinatal Gdf11−/−;Vsx2orJ/orJ mice indicates that a significant number of axons are present in an (aberrant) optic nerve, and that these axons extend as far as the optic chiasm (data not shown). These observations suggest that the rescue of retinal development seen in these animals may extend to a restoration of visual function.
Altogether, these studies show that manipulation of the GDF11 signaling pathway can strongly influence the severity of developmental retinal dystrophies, and suggest that pharmacological intervention with the GDF11 signaling pathway may be a potent means by which to treat retinal dystrophies. To this end, current studies are aimed at understanding the molecular mechanisms by which rescue is achieved.
Acknowledgments
We thank T. Clevenger, R. Asperer, C. Yaramanoglu, and M. Yazdi for technical assistance and K. Gokoffski, S. Kawauchi, and P. Hollenbeck for comments on the manuscript. This study was supported by grants from the Foundation Fighting Blindness (BR-CMM-0507-0380-UCI) and NIH (DC03583, GM076516) to ALC, and MRC G0601182 to RH. RS received the Young Investigator Travel Award by the National Eye Institute (NIH) to attend the XIVth International Symposium on Retinal Degeneration 2010.
Contributor Information
Rosaysela Santos, Department of Anatomy & Neurobiology, Center for Complex Biological Systems, University of California, Irvine, CA 92697-1275, USA.
Jeffry Wu, Department of Anatomy & Neurobiology, Center for Complex Biological Systems, University of California, Irvine, CA 92697-1275, USA; BOOPT-School of Optometry, University of California, Berkeley, CA, 94720-2284, USA.
Jason A. Hamilton, Department of Anatomy & Neurobiology, Center for Complex Biological Systems, University of California, Irvine, CA 92697-1275, USA; Department of Regenerative Medicine, Athersys Inc, 3021 Carnegie Ave., Cleveland, OH 44115-2634, USA
Rita Pinter, MRC Centre for Developmental Neurobiology, Kings College, London, SE1 1UL, UK; Akron Molecules GmbH, Campus Vienna Biocenter 5, Vienna, 1030, Austria.
Robert Hindges, MRC Center for Developmental Neurobiology, Kings College, London, SE1 1UL, UK.
Anne L. Calof, Email: alcalof@uci.edu, Department of Anatomy & Neurobiology, Center for Complex Biological Systems, University of California, Irvine, CA 92697-1275, USA.
References
- Barabino GA, Wise RJ, Woodbury VA, et al. Inhibition of sickle erythrocyte adhesion to immobilized thrombospondin by von Willebrand factor under dynamic flow conditions. Blood. 1997;89:2560–2567. [PubMed] [Google Scholar]
- Burmeister M, Novak J, Liang MY, et al. Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nat Genet. 1996;12:376–384. doi: 10.1038/ng0496-376. [DOI] [PubMed] [Google Scholar]
- Erkman L, McEvilly RJ, Luo L, et al. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature. 1996;381:603–606. doi: 10.1038/381603a0. [DOI] [PubMed] [Google Scholar]
- Ferda Percin E, Ploder LA, Yu JJ, et al. Human microphthalmia associated with mutations in the retinal homeobox gene CHX10. Nat Genet. 2000;25:397–401. doi: 10.1038/78071. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Li T, et al. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat Genet. 1999;23:466–470. doi: 10.1038/70591. [DOI] [PubMed] [Google Scholar]
- Harada T, Harada C, Parada LF. Molecular regulation of visual system development: more than meets the eye. Genes Dev. 2007;21:367–378. doi: 10.1101/gad.1504307. [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Kim J, Santos R, et al. Foxg1 promotes olfactory neurogenesis by antagonizing Gdf11. Development. 2009;136:1453–1464. doi: 10.1242/dev.034967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly LE, Nekkalapudi S, El-Hodiri HM. Expression of the forkhead transcription factor FoxN4 in progenitor cells in the developing Xenopus laevis retina and brain. Gene Expr Patterns. 2007;7:233–238. doi: 10.1016/j.modgep.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Wu HH, Lander AD, et al. GDF11 controls the timing of progenitor cell competence in developing retina. Science. 2005;308:1927–1930. doi: 10.1126/science.1110175. [DOI] [PubMed] [Google Scholar]
- Lander AD, Gokoffski KK, Wan FY, et al. Cell lineages and the logic of proliferative control. PLoS Biol. 2009;7:e15. doi: 10.1371/journal.pbio.1000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RC, Navi D, Fesenko J, et al. Widespread defects in the primary olfactory pathway caused by loss of Mash1 function. J Neurosci. 2003;23:1769–1780. doi: 10.1523/JNEUROSCI.23-05-01769.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan S, Chen CM, Young TL, et al. Transdifferentiation of the retina into pigmented cells in ocular retardation mice defines a new function of the homeodomain gene Chx10. Development. 2004;131:5139–5152. doi: 10.1242/dev.01300. [DOI] [PubMed] [Google Scholar]
- Wu HH, Ivkovic S, Murray RC, et al. Autoregulation of neurogenesis by GDF11. Neuron. 2003;37:197–207. doi: 10.1016/s0896-6273(02)01172-8. [DOI] [PubMed] [Google Scholar]
- Xuan S, Baptista CA, Balas G, et al. Winged helix transcription factor BF-1 is essential for the development of the cerebral hemispheres. Neuron. 1995;14:1141–1152. doi: 10.1016/0896-6273(95)90262-7. [DOI] [PubMed] [Google Scholar]


