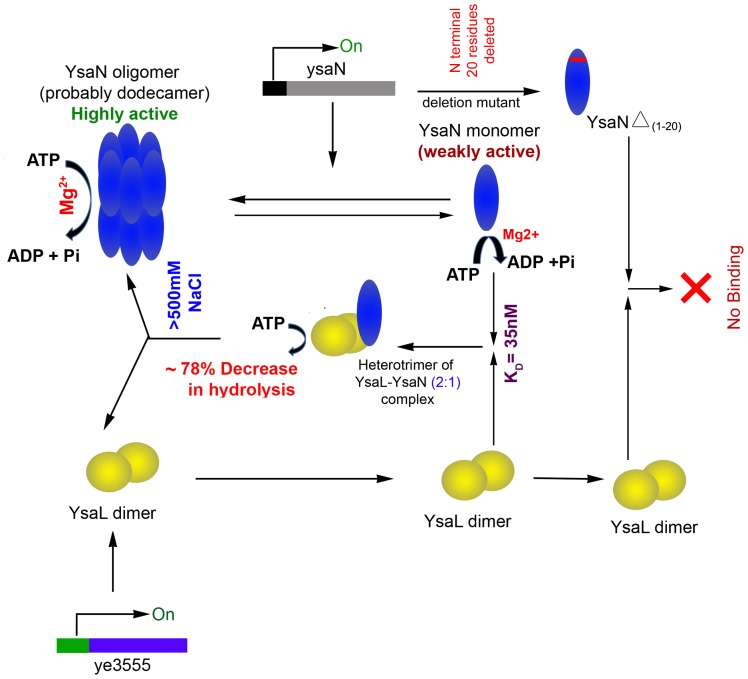
Figure 6. Schematic representation of functionality of YsaN and its regulation by YsaL.
YsaN exists in solution as mixture of monomer and higher order oligomer (dodecamer) and act as Mg 2+ dependent ATPase. The oligomeric form of YsaN is highly active compared to monomeric form. Computational studies predicted YsaL as a putative ATPase regulator. YsaL exists as dimer in solution and form stable heterotrimeric complex with monomeric YsaN. This results in significantly loss of ATPase activity of YsaN, probably due to loss of oligomeric state. The complex is unstable at high salt concentration (>500 mM NaCl). Furthermore, N-terminal (1–20) residues of YsaN are involved in YsaL-YsaN interaction, as revealed from interaction studies of deletion mutants.