Fig 2.
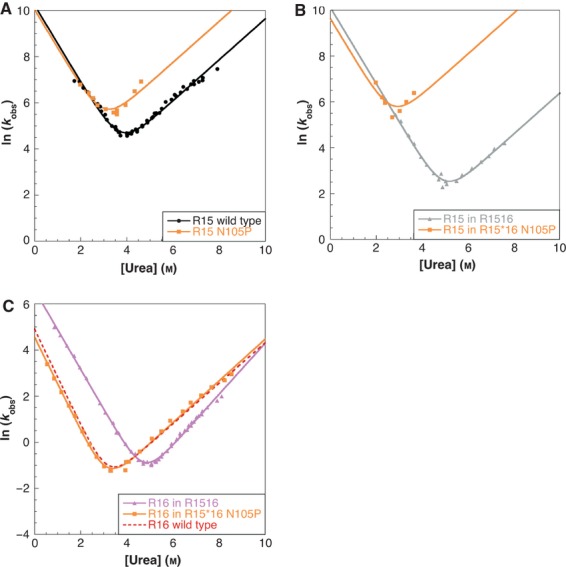
The mutation N105P in R15 results in loss of stabilizing interactions between the domains in R1516. (A) Single domains: WT R15 (black) and R15 N105P (orange). The folding rate is essentially unaffected by the mutation; however, the mutation causes an increase in the unfolding rate of R15. (B) R15 in WT R1516 (grey) and mutant R1516 (orange). The mutation affects the R15 domain exactly as in the single-domain protein. The unfolding rate of the mutant is now significantly faster than WT R15 in R1516. (C) R16 in WT R1516 (pink) and mutant R1516 (orange). The effect is dramatic. The mutant protein folds much more slowly and unfolds faster than WT. In fact, it folds just like the WT R16 single-domain protein. Thus folding of R16 in the mutant R1516 is essentially identical to the WT form (included for comparison, red dotted line). All the stabilizing interactions between R15 and R16 have been lost.
