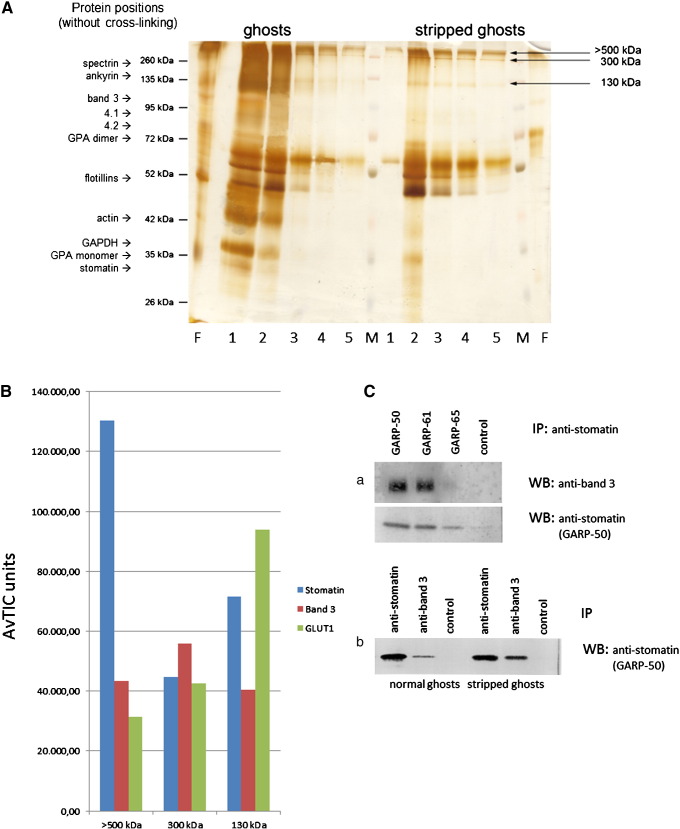
Fig. 1.
Isolation and analysis of EGS-cross-linked stomatin complexes and immunochemical verification of stomatin-band 3 interaction. (A) Erythrocyte membranes (“ghosts”), either untreated (left side) or depleted of cytoskeleton (“stripped ghosts”, right side), were cross-linked with 0.8 mM EGS, quenched, and solubilised; stomatin-complexes were isolated by immunoaffinity chromatography. Elution fractions were analysed by 10% SDS-PAGE/silver staining, excision of bands, as indicated, and mass spectrometry. Positions of un-cross-linked erythrocyte membrane proteins are indicated. Flow-through and elution fractions are shown. (B) Three stomatin-complexes isolated from stripped ghosts, > 500 kDa, about 300 kDa, and 130 kDa, contain stomatin, band 3, and GLUT1 as major components. Semi-quantitative results are shown as Average Total Ion Current (AvTIC) units. (C) Verification of the stomatin-band 3 interaction. (a) IP/WB analysis with monoclonal anti-stomatin antibodies against N- (GARP-50) and C-terminal (GARP-61, -65) epitopes and monoclonal anti-band 3, as indicated (GARP-65 has weak affinity). Control is an irrelevant antibody. (b) IP/WB analysis using normal or stripped ghosts. Co-IP works better with stripped ghosts. M, marker; F, flow-through; 1–5, respective elution fractions 1–5; GPA, glycophorin A; IP, immunoprecipitation; WB, Western blotting.