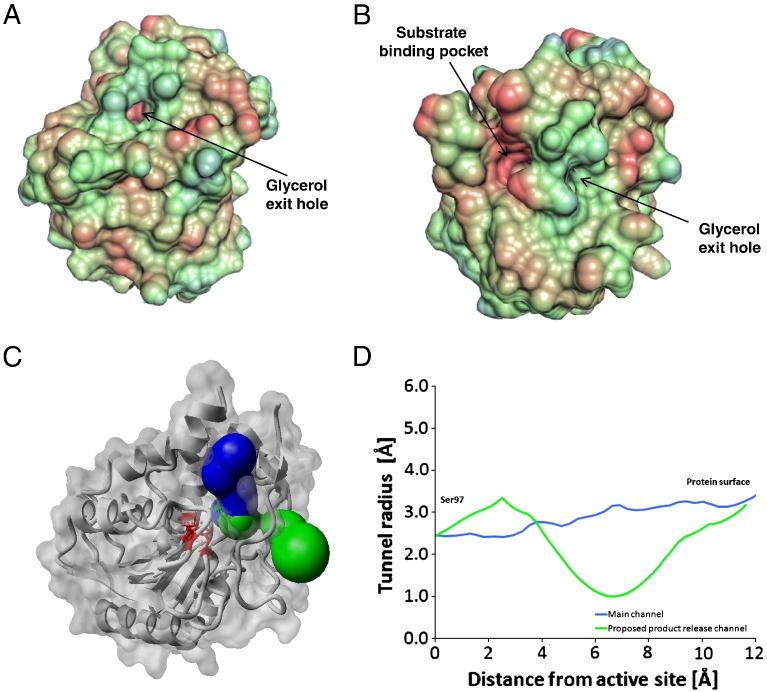
Fig. 3.
Surface and access paths to the catalytic site of bMGL. A and B) Surface representations of the uncomplexed bMGL structure showing the substrate-binding pocket and the glycerol exit hole in two different orientations. The colouring scheme represents the lipophilic potential: Regions coloured red are hydrophobic, blue are hydrophilic. A) Orientation of the protein as in Fig. 1. B) Orientation of the protein after rotation of 55 and 87° along X and Y axes, respectively, for better visualisation of the substrate binding pocket. C) Access paths to the active site of bMGL. The main active site channel is shown in blue, and the proposed glycerol exit hole is shown in green. Residues shown as red sticks are part of the catalytic triad (orientation of the molecule similar to Fig. 3B). D) Radii profile of the access paths of bMGL starting from the active site (Ser97) towards the protein surface.