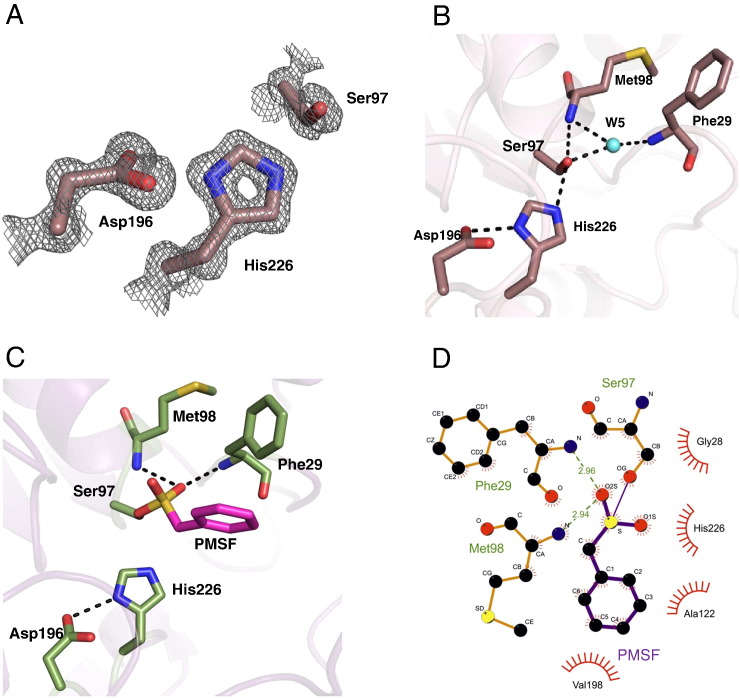
Fig. 4.
Active site architecture of bMGL. A) The 2Fo–Fc sigma weighted electron density map (grey) is contoured at 1σ around the catalytic triad residues Ser97, Asp196 and His226 (violet sticks). B) The catalytic triad environment in the presence of a water molecule in the uncomplexed structure. H-bonds are depicted as dashed lines. The catalytic triad and oxyanion hole residues are shown in violet. The water molecule (W5) mediating the polar contacts is depicted in cyan. C) Environment of the catalytic triad in the bMGL–PMSF complex structure. H-bonds are depicted as dashed lines. The catalytic triad residues and oxyanion hole forming residues are shown in green. The phenyl group of the PMSF molecule covalently bound to Ser97 is represented in magenta. D) Depiction of atomic details of the interaction between bMGL and PMSF using LigPlot + [60].