Abstract
Background
Over recent years, use of the LigaSure™ vessel sealing device has increased in major abdominal surgery to include pancreaticoduodenectomy (PD). LigaSure™ use during PD has expanded to include all steps of the procedure, including the division of the uncinate margin. This introduces the potential for thermal major vascular injury or margin positivity. The aim of the present study was to evaluate the safety and efficacy of LigaSure™ usage in PD in comparison to established dissection techniques.
Methods
One hundred and forty-eight patients who underwent PD from 2007 to 2012 at Robert Wood Johnson University Hospital were identified from a retrospective database. Two groups were recognized: those in which the LigaSure™ device was used (N = 114), and in those it was not (N = 34). Peri-operative outcomes were compared.
Results
Vascular intra-operative complications directly caused by thermal injury from LigaSure™ use occurred in 1.8% of patients. Overall vascular intra-operative complications, uncinate margin positivity, blood loss, length of stay, and complication severity were not significantly different between groups. The mean operative time was 77 min less (P < 0.010) in the LigaSure™ group. Savings per case where the LigaSure™ was used amounted to $1776.73.
Conclusion
LigaSure™ usage during PD is safe and effective. It is associated with decreased operative times, which may decrease operative costs in PD.
Introduction
For decades, pancreaticoduodenectomy (PD), i.e. the Whipple procedure, and the later-established pylorus-preserving PD have remained the standard operations for resecting peri-ampullary tumours, but are also indicated in benign disorders such as chronic pancreatitis and pancreatic cyst disease.1 The operation is technically challenging in part as a result of the extensive dissection necessary for the resection and the location of the pancreas near major vasculature. There are additional oncological challenges particularly with obtaining a negative uncinate (or retroperitoneal) margin, as this step requires dissection of the uncinate process from the superior mesenteric artery (SMA). There are various techniques described to perform this step, including the clamp and cut, endovascular stapler, and ultrasonic dissector.2 While no specific technique has proven superior, the margin positivity rates remain highly variable, ranging from 16% to 85% in some studies.2–4
The development of the LigaSure™ (ValleyLab) bipolar device has provided a different means of achieving haemostasis via sealing vessels by melting collagen and elastin.5,6 Use of the LigaSure™ device has increased in major abdominal surgery over recent years to include hepatic surgery, where its use has been described in hepatic parenchymal transection.7 Prior to 2011, one case report examined its use as the primary means for haemostasis in PD,8 and another study described its safety in isolated use during jejunal resection in PD.9 Recently, LigaSure™ usage has been evaluated in PD in a small-sample pilot study that showed a decrease in blood loss and operative time in PD when comparing LigaSure™ usage with established dissection techniques.10 However, both the safety of LigaSure™ use pertaining to thermal major vascular injury and efficacy pertaining to uncinate margin positivity were not reported. Given the apparent increase in use of this device in PD, the aim of this study was to evaluate both the safety and efficacy of LigaSure™ usage in PD in comparison to established dissection techniques.
Methods
Patient selection and analysis
Analysis was based on data acquired from an institutional review board-approved database and electronic medical records at Robert Wood Johnson University Hospital (New Brunswick, NJ, USA). A total of 148 patients who underwent PD from July 2007 to May 2012 were identified. All of the operations were performed by three surgeons over this time period, composed of one senior surgeon and two junior faculty members. The LigaSure™ device used in all cases but five was the LigaSure Impact™ hand piece instrument (13.5-mm shaft diameter, jaw angle curved 14 degrees). In the remaining five cases, the LigaSure Small Jaw™ was used. Upon review of operative records, patients were placed into one of two groups: cases in which the LigaSure™ device was primarily used for dissection and haemostasis (N = 114), and cases in which it was not (N = 34). In six of the patients in the non-LigaSure™ (NL) group, the LigaSure™ device was minimally used during each operation, either to mobilize the jejunum or to facilitate entering the lesser sac, and not as the primary means for dissection and haemostasis. In none of those cases was the LigaSure™ device used around the superior mesenteric vein (SMV), SMA, portal vein (PV) or in uncinate margin transection. Vascular intra-operative complications were identified from operative reports and discussions with operating surgeons, as well as any thermal injuries directly as a result of LigaSure™ usage. We defined a vascular intra-operative complication as an injury to a major vessel (SMV, SMA and PV) or direct branch thereof. The uncinate margin positivity rate, estimated blood loss, operative time, complications and length of stay were compared between the groups. Statistical analysis included Student's t-tests for continuous variables, chi-squared tests for categorical data, and Wilcoxon–Mann–Whitney rank sum tests for ordinal variables.
LigaSure™ versus established techniques
PD technique was not standardized among surgeons. Established techniques for dissection and haemostasis involved clips, ties, scissors, and suture ligatures. In cases where the LigaSure™ was used, the instrument was utilized in various aspects of the operation but most crucially the dissection around the PV, SMV and SMA. The second most common step in the operation in which the LigaSure™ was used was during the division of the mesenteric vessels of the proximal jejunum and third/fourth portion of the duodenum. In this study, particular attention was given to the step in the operation during dissection of the uncinate from the SMA, as the use of the device in this step has the potential for thermal injury to the adjacent major vasculature (PV/SMV), and this is the crucial step that can potentially influence the margin, particularly in cases of pancreatic adenocarcinoma.
Results
The pattern of LigaSure™ use (Fig. 1) indicates that by 2010, the LigaSure™ was being used routinely for all aspects of the dissection, including the uncinate margin, by all three surgeons. From 2007 to 2009, LigaSure™ usage has steadily increased, reflecting a gradual shift in preference for use of the device. During this earlier period, the selection of LigaSure™ was not influenced by any particular factor, including histology, patient factors, or level of complexity of the case.
Figure 1.
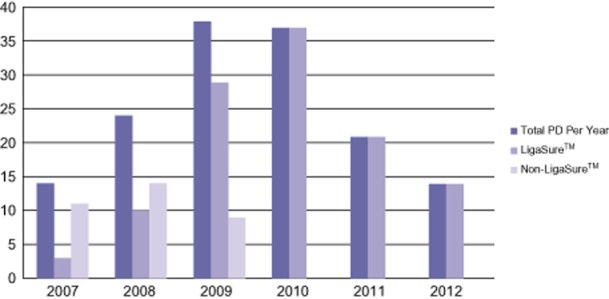
Evolution of LigaSure™ usage in pancreaticoduodenectomy (PD)
Table 1 shows demographics and diagnoses of patients in each group. There were no significant differences in the comorbidities and pre-operative albumin between the two groups. Likewise, the proportion of patients with a diagnosis of pancreatic adenocarcinoma between the two groups was similar (P = 0.631). Of patients diagnosed with pancreatic adenocarcinoma, the percentage of patients who underwent neoadjuvant therapy was similar between the groups (P = 0.945).
Table 1.
Patient group demographics
| LigaSure™ | Non-LigaSure™ | P-value | |
|---|---|---|---|
| Number of patients | 114 | 34 | |
| Mean age (range) | 64.4 (38–88) | 61 (13–83) | 0.287 |
| Number of females (%) | 62 (54.4) | 15 (44.1) | 0.293 |
| Past medical history, n (%) | |||
| Coronary artery disease | 14 (12.3) | 6 (17.6) | 0.422 |
| Chronic obstructive pulmonary disease, asthma, and/or obstructive sleep apnoea | 18 (15.8) | 6 (17.6) | 0.797 |
| Diabetes mellitus | 39 (34.2) | 11 (32.4) | 0.841 |
| Smoking | 31 (27.2) | 5 (14.7) | 0.136 |
| Mean pre-operative serum albumin level, g/dl (range) | 3.5 (1.8–4.5) | 3.4 (0.6–4.5) | 0.328 |
| Neoadjuvant therapy (pancreatic adenocarcinoma), n (%) | 7 (6.1) | 2 (5.9) | 0.945 |
| Diagnosis, n (%) | |||
| Adenocarcinoma | 73 (64.0) | 21 (61.8) | |
| Pancreatic | 59 (51.8) | 16 (47.1) | 0.631 |
| Duodenal | 12 (10.5) | 4 (11.8) | |
| Common bile duct | 2 (1.8) | 1 (2.9) | |
| Intraductal papillary mucinous neoplasm | 12 (10.5) | 4 (11.8) | |
| Endocrine neoplasm | 8 (7.0) | 2 (5.9) | |
| Other tumours | 8 (7.0) | 6 (17.6) | |
Table 2 compares the peri-operative outcomes between the two groups. Vascular intra-operative complications directly as a result of thermal injury from LigaSure™ use occurred in 2/114 patients (1.8%). The first case occurred in a patient with pancreatic adenocarcinoma previously explored for resection, but was aborted due to SMV/PV involvement with a Roux-en-Y hepaticojejunostomy and gastrojejunostomy created. That patient then underwent chemotherapy and radiation. Repeat imaging indicated resectability, and the patient was brought to the operating room for a second attempt at resection. It was during this operation that a thermal injury to the PV occurred during the dissection of the uncinate margin. This injury was created using the LigaSure™ on the uncinate margin tissue parallel to the PV, without retraction of the PV. Vascular control was obtained with suture ligatures, but the patient became haemodynamically unstable, and the case was aborted. This patient subsequently died of multisystem organ failure. In the second case, the LigaSure™ was used for dissection around the uncinate process and created a thermal injury on the main jejunal tributary to the superior mesenteric vein. After a significant haemorrhage, control was obtained with suture ligatures. The patient remained haemodynamically stable during the event and for the entirety of the case.
Table 2.
Peri-operative outcomes
| LigaSure™ | Non-LigaSure™ | P-value | |
|---|---|---|---|
| Number of patients | 114 | 34 | |
| Vascular intra-operative complications, n (%) | 6 (5.3) | 5 (14.7) | 0.065 |
| Thermal injury caused by LigaSure™, n (%) | 2 (1.8) | – | |
| Uncinate margin positivity for pancreatic adenocarcinoma, n (%) | 12 (20.3) | 4 (25.0) | 0.687 |
| Venous resection, n (%) | 10 (8.8) | 2 (5.9) | 0.588 |
| Mean estimated blood loss, ml (range) | 926 (100–15000) | 1163 (150–4000) | 0.279 |
| Mean operative time, min (range) | 416 (232–779) | 493 (313–767) | <0.010 |
| Median length of stay, days (range) | 8 (4–68) | 11.5 (5–66) | 0.186 |
| Clavien–Dindo classification at 90 days, n (%) | |||
| Clavien 0 | 35 (30.7) | 11 (32.4) | 0.855 |
| Clavien 1–2 | 42 (36.8) | 13 (38.2) | 0.883 |
| Clavien 3–5 | 37 (32.5) | 10 (29.4) | 0.738 |
| Mean Clavien classification at 90 days (range) | 1.9 (1–5) | 1.8 (1–5) | 0.717 |
Overall, vascular intra-operative complications occurred in 6 LigaSure™ patients and 5 NL patients (P = 0.065). Venous resection was performed in 10 LigaSure™ patients and 2 NL patients (P = 0.588). Uncinate margin positivity for pancreatic adenocarcinoma was not significantly different between the groups (P = 0.687). Mean estimated blood losses were 926 and 1163 ml (P = 0.279), and mean operative times were 416 and 493 min, (P < 0.010) in LigaSure™ and NL patients, respectively. Median lengths of stay in days were 8 for LigaSure™ patients and 11.5 for NL patients (P = 0.186). The mean post-operative Clavien–Dindo complication severity grades at 90 days were 1.9 and 1.8 in LigaSure™ patients and NL patients, respectively (P = 0.717). Detailed post-operative complications are shown in Table 3. The pancreatic anastomotic leak/abscess/fistula rate was not significantly different between the groups (P = 0.936). Thirty-day mortality was not significantly different between the groups (P = 0.446).
Table 3.
Post-operative complications, n (%)
| LigaSure™ | Non-LigaSure™ | |
|---|---|---|
| Number of patients | 114 | 34 |
| Cardiovascular | 19 (16.7) | 7 (20.6) |
| Pulmonary | 16 (14.0) | 9 (26.5) |
| Gastrointestinal | 42 (36.8) | 17 (50.0) |
| Liver | 7 (6.1) | 2 (5.9) |
| Pancreas | 20 (17.5) | 4 (11.8) |
| Anastomotic leak/abscess/fistula, P = 0.936 | 14 (12.3) | 4 (11.8) |
| Other infections | 34 (29.8) | 9 (26.5) |
| Miscellaneous | 8 (7.0) | 1 (2.9) |
| 30-day mortality, P = 0.446 | 6 (5.3) | 3 (8.8) |
| 90-day mortality | 5 (4.4) | – |
| Total | 157 | 52 |
| Complications per patient | 1.4 | 1.5 |
Discussion
The current literature examining LigaSure™ usage in PDs is sparse.8,9 A recent pilot case–control study10 examined the safety of LigaSure™ usage in seven patients undergoing PD compared with seven via conventional techniques. Significant decreases in operative time, blood loss and costs were found when the LigaSure™ device was used. Given the small size of this study, it is difficult to make a reliable assessment of the safety and efficacy of the instrument in PD. These same authors have initiated a larger prospective randomized study to more rigorously evaluate the device;11 however at this time, there are no other studies that have examined both the safety and efficacy of this device specifically in PD.
LigaSure™ usage has been increasing in major abdominal surgery, with various studies reporting on its safety.6,7,12,13 In one study examining the safety of LigaSure™ dissection in Ivor-Lewis esophagectomies compared with established techniques, LigaSure™ usage was associated with a significant decrease in operative time and blood loss.12 Such results have been reported in gastric and colorectal cancer operations with extended lymph node dissections as well.13 LigaSure™ usage is also safe during hepatic parenchyma dissection.7 The LigaSure™ device has even been shown to produce decreased lateral thermal damage on the human peritoneum compared with monopolar diathermy.14
Use of the LigaSure™ device is a safe and effective alternative to established dissection techniques in PD. It is associated with decreased operative times and a similar uncinate margin positivity rate. While not significantly different, we did find that LigaSure™ usage was associated with over 200 ml less blood loss intra-operatively, a decreased intra-operative vascular injury rate, and a 3.5-day shorter length of hospital stay. These measured variables for both the LigaSure™ and non-LigaSure™ groups were similar to established norms for peri-operative complications and length of stay.15–17
The decision to evaluate the LigaSure™ in PD is based upon the increased use of the device during the surgery over time as shown in Fig. 1. As experience with it has grown, so too has its application to virtually all steps in the dissection, including the uncinate margin. This is a crucial step in the operation where there is potential for thermal injury to the PV/SMV. These data would indicate that major vascular injury, particularly PV/SMV injury, was not statistically significantly different when using the LigaSure™ compared with NL techniques. Two vascular injuries occurred directly from thermal injury with the use of the device, and we feel this is important to report. As experience using the LigaSure™ has grown, techniques have been developed to help avoid such types of injury, and these have been illustrated in Figs 2–4. Nonetheless, one of these complications was a death that occurred in the setting of a re-exploration after radiation. Thus, for the surgeon just starting to incorporate the LigaSure™ in the uncinate dissection, we would discourage the use of the LigaSure™ in this portion of the operation for complex cases such as this.
Figure 2.
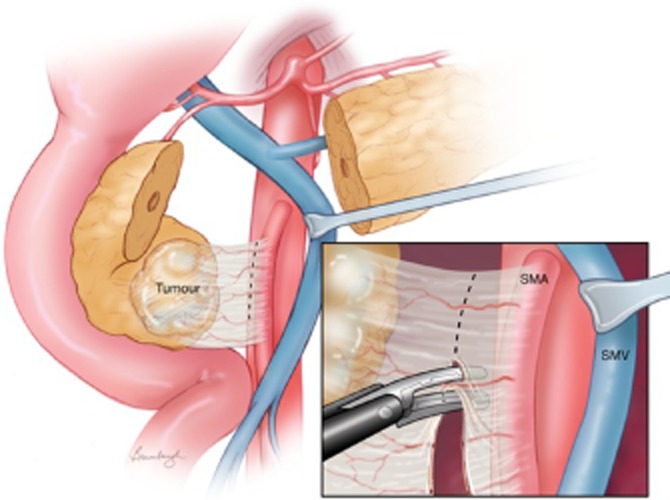
Proper technique: during uncinate margin dissection, a thermal injury can occur as the LigaSure™ comes in close contact with the portal and superior mesenteric vein (SMV) confluence. It is essential to retract the vein as depicted here. Division of the uncinate margin can be done taking perpendicular bites as indicated here, or by placing the device parallel to the superior mesenteric artery (SMA)
Figure 4.
Use of the LigaSure™ to seal vessels of the proximal jejunum and duodenum after division of the jejunum. These vessels are small and numerous, with ligation and division by conventional ties tedious. Use of the LigaSure™ greatly speeds up this step of the operation
Figure 2 demonstrates the proper technique to apply when using the LigaSure™ in the uncinate margin dissection. The SMV/PV is dissected from the pancreas, and the vein is retracted with either a vessel loop or vein retractor. The LigaSure™ can be used either perpendicular to the tissue along the SMA or parallel to it. This provides the optimal method of maintaining haemostasis and minimizing thermal injury. The improper technique is shown in Fig. 3, in which the LigaSure™ is used without retraction of the SMV/PV. Figure 4 illustrates the use of the LigaSure™ device in dividing mesenteric vessels after division of the jejunum.
Figure 3.
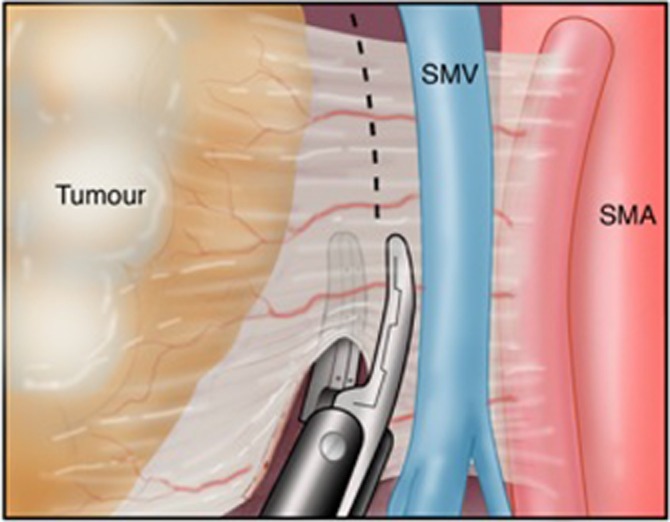
Improper technique: parallel positioning of the LigaSure™ without retracting the vein while dividing tissue at the uncinate margin. SMV, superior mesenteric vein; SMA, superior mesenteric artery
The other reason to investigate the device at this step of the operation is because of its potential impact on the uncinate margin positivity rate. The uncinate margin positivity rate in different series of PD has varied significantly.2–4 There are likely several reasons for this, such as the inclusion of patients with all types of peri-ampullary tumours, patients having undergone neoadjuvant therapy, and a lack of standard dissection techniques. Given these factors, we chose to limit our analysis of the uncinate margin positivity rate to only those patients undergoing the operation for pancreatic adenocarcinoma. There was no difference between the two groups in the percentage of patients with a diagnosis of pancreatic adenocarcinoma, and no difference in the uncinate margin positivity rate between the LigaSure™ and NL groups, both of which fell within reported rates in the literature.2–4 It is our practice to use neoadjuvant therapy in patients with borderline or locally advanced tumours; the majority of our patients (88%) with pancreatic adenocarcinoma did not receive pre-operative therapy. With respect to the different techniques to transect the uncinate margin, our group of non-LigaSure™ techniques was fairly uniform with the majority using clamps and ties and only one case where the vascular stapler was used.
It was found that the use of the LigaSure™ in PD is associated with a significant decrease in operative time when compared with the NL group. The mean operative time was 77 min shorter in the LigaSure™ versus NL group. Our findings are consistent not only with reports of the device in PDs but in other major abdominal surgery.6,7,13 At our institution, as demonstrated in Table 4, this would save $2246.09 per case and more than offset the cost of the device ($472.42), with mean overall savings per case of $1773.67. Gehrig et al. also found that the use of the device resulted in reduced blood loss.10 These data indicated a trend towards this finding but was not statistically significant.
Table 4.
Operative costs
| LigaSure™ | Non-LigaSure™ | |
|---|---|---|
| Mean operative time, min | 416 | 493 |
| Mean operative cost, $ | 12134.72 | 14380.81 |
| Mean savings per case, $ | 2246.09 | |
| LigaSure™ cost per case, $ | 472.42 | |
| Mean overall savings per case, $ | 1773.67 | |
We acknowledge several weaknesses of this study, the first being its retrospective design. Given the rapid adoption of this technique at this institution, we had a relatively small group of NL patients to compare. We could have gone back further in time to collect data on more patients in whom the device was not used, however, those cases were done by a different group of surgeons, and this would have introduced another element of bias. Due to increasing LigaSure™ usage at this institution over time, experience may have contributed to the decreased operative times. Two of the three surgeons were junior faculty members and in the early years of practice. We acknowledge that the learning curve of these surgeons in performing a PD is a complicating factor, and improvement in operative times could very well have been influenced by this learning curve. Of note, however, the senior surgeon in this group has over two decades of experience performing PD, and he performed the majority of cases in this dataset, both overall (54.4%) and in the NL group (73.5%). Thus, the longer operative times observed in the NL group were less likely influenced by the factor of surgeon experience.
While our pancreatic anastomotic leak rates were not significantly different between the two groups, data regarding risk stratification of anastomotic leaks, including gland texture and duct size, were not routinely mentioned in operative reports. However, blood loss and histology are two factors that have been identified as independent predictors of a pancreatic anastomotic leak,18 and our data demonstrates no significant difference between the two groups. Nonetheless, a prospective randomized trial is underway in Europe to evaluate the use of the LigaSure™ in PD, and we look forward to this report.11
LigaSure™ usage in PD is safe, effective, and is associated with decreased operative time. The present study incorporates the largest population of PD patients to date in examining the safety of LigaSure™ usage, and is the first to report on its efficacy during uncinate margin transection in patients with pancreatic adenocarcinoma.
Conflicts of interest
None declared.
References
- 1.Diener MK, Fitzmaurice C, Schwarzer G, Seiler CM, Antes G, Knaebel HP, et al. Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database Syst Rev. 2011;(5) doi: 10.1002/14651858.CD006053.pub4. CD006053. [DOI] [PubMed] [Google Scholar]
- 2.Katz MH, Merchant NB, Brower S, Branda M, Posner MC, William Traverso L, et al. Standardization of surgical and pathologic variables is needed in multicenter trials of adjuvant therapy for pancreatic cancer: results from the ACOSOG Z5031 trial. Ann Surg Oncol. 2011;18:337–344. doi: 10.1245/s10434-010-1282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raut CP, Varadhachary G, Wang H, Tamm EP, Fleming JB, Evans DB. Margin status following pancreaticoduodenectomy for pancreatic adenocarcinoma: implications of R status. In: Beger HG, Matsuno S, Cameron JL, Rau BM, Sunamura M, Schulick RD, editors. Diseases of the Pancreas. Berlin: Springer; 2008. pp. 611–623. [Google Scholar]
- 4.Zhang Y, Frampton AE, Cohen P, Kyriakides C, Bong JJ, Habib NA, et al. Tumor infiltration in the medial resection margin predicts survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J Gastrointest Surg. 2012;16:1875–1882. doi: 10.1007/s11605-012-1985-4. [DOI] [PubMed] [Google Scholar]
- 5.Heniford BT, Matthews BD, Sing RF, Backus C, Pratt B, Greene FL. Initial results with an electrothermal bipolar vessel sealer. Surg Endosc. 2001;15:799–801. doi: 10.1007/s004640080025. [DOI] [PubMed] [Google Scholar]
- 6.Janssen PF, Brolmann HA, Huirne JA. Effectiveness of electrothermal bipolar vessel-sealing devices versus other electrothermal and ultrasonic devices for abdominal surgical haemostasis: a systematic review. Surg Endosc. 2012;26:2892–2901. doi: 10.1007/s00464-012-2276-6. [DOI] [PubMed] [Google Scholar]
- 7.Patrlj L, Tuorto S, Fong Y. Combined blunt-clamp dissection and LigaSure ligation for hepatic parenchyma dissection: postcoagulation technique. J Am Coll Surg. 2010;210:39–44. doi: 10.1016/j.jamcollsurg.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 8.Belli G, Fantini C, Ciciliano F, D'Agostino A, Barberio M. Pancreaticoduodenectomy in portal hypertension: use of the Ligasure. J Hepatobiliary Pancreat Surg. 2003;10:215–217. doi: 10.1007/s00534-002-0745-3. [DOI] [PubMed] [Google Scholar]
- 9.Howard TJ, Mimms S. Use of a new sealing device to simplify jejunal resection during pancreaticoduodenectomy. Am J Surg. 2005;190:504–506. doi: 10.1016/j.amjsurg.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 10.Gehrig T, Muller-Stich BP, Kenngott H, Fischer L, Mehrabi A, Buchler MW, et al. LigaSure versus conventional dissection technique in pancreatoduodenectomy: a pilot study. Am J Surg. 2011;201:166–170. doi: 10.1016/j.amjsurg.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Gehrig T, Knebel P, Scheel V, Hinz U, Seiler CM, Muller-Stich BP, et al. LigaSure impact versus conventional dissection technique in pylorus-preserving pancreatoduodenectomy in clinical suspicion of cancerous tumours on the head of the pancreas: study protocol for a randomised controlled trial. Trials. 2011;12:1–8. doi: 10.1186/1745-6215-12-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sayir F, Cobanoglu U, Sehitogullari A. The use of LigaSure Vessel Sealing System in Ivor Lewis esophagectomy. J Cardiothorac Surg. 2012;7:1–7. doi: 10.1186/1749-8090-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takiguchi N, Nagata M, Soda H, Nomura Y, Takayama W, Yasutomi J, et al. Multicenter randomized comparison of LigaSure versus conventional surgery for gastrointestinal carcinoma. Surg Today. 2010;40:1050–1054. doi: 10.1007/s00595-009-4234-z. [DOI] [PubMed] [Google Scholar]
- 14.Druzijanic N, Pogorelic Z, Perko Z, Mrklic I, Tomic S. Comparison of lateral thermal damage of the human peritoneum using monopolar diathermy, Harmonic scalpel and LigaSure. Can J Surg. 2012;55:317–321. doi: 10.1503/cjs.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gouma DJ, van Geenen RC, van Gulik TM, de Haan RJ, de Wit LT, Busch OR, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000;232:786–795. doi: 10.1097/00000658-200012000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt CM, Powell ES, Yiannoutsos CT, Howard TJ, Wiebke EA, Wiesenauer CA, et al. Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg. 2004;139:718–725. doi: 10.1001/archsurg.139.7.718. discussion 25–7. [DOI] [PubMed] [Google Scholar]
- 18.Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM., Jr A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216:1–14. doi: 10.1016/j.jamcollsurg.2012.09.002. [DOI] [PubMed] [Google Scholar]


