Figure 8. Schematic representation of caveolin-mediated cytoskeletal regulation in microglia cells.
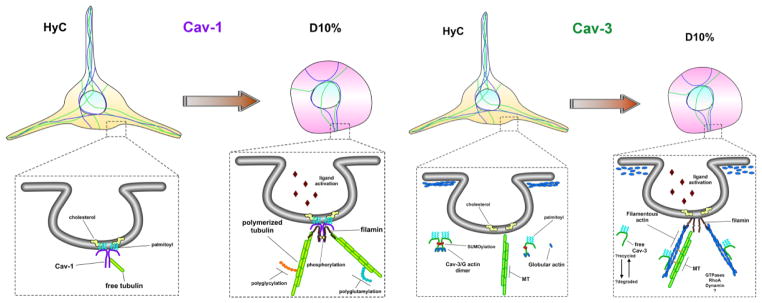
In quiescent microglia, represented by our HyC condition, Cav-1 resides within the inner leaflet of the PM, anchored to cholesterol via palmitoylated cysteine residues and interacts with free-non-polymerized tubulin to maintain a readily available pool, while the majority of cellular tubulin is polymerized to maintain the long and active sentinel processes. Once caveolae-mediated receptors bind ligand, represented by our D10% condition, free tubulin is displaced, by another microtubule binding partner, possibly filamin, which tethers the now polymerizing microtubules to the internalizing caveolae, facilitating downstream signaling cascades. Cav-3, on the other hand, is never localized to the PM and remains within the cytosol, as monomers or dimers, or bound to actin cytoskeletal compartments. Unbound free cytosolic Cav-3 interacts with, and maintains a pool of G-actin monomers, near the inner leaflet of the PM, which is structurally maintained by a dense cortical actin network, represented by HyC cells. Once caveolae-mediated receptors bind ligand, the G-actin is released by the cytosolic Cav-3 or directly incorporated, along with Cav-3, into the rapidly nucleating actin needed for internal transport. The released cytosolic Cav-3 is either degraded or is available to interact with the rapidly denucleating cortical actin network G-actin monomers, to resupply the localized environment for further signaling.
