Abstract
Nonalcoholic fatty liver disease is the most common liver disease in both adults and children. The earliest stage of this disease is hepatic steatosis, in which triglycerides are deposited as cytoplasmic lipid droplets in hepatocytes. Through a forward genetic approach in zebrafish, we found that guanosine monophosphate (GMP) synthetase mutant larvae develop hepatic steatosis. We further demonstrate that activity of the small GTPase Rac1 and Rac1-mediated production of reactive oxygen species (ROS) are down-regulated in GMP synthetase mutant larvae. Inhibition of Rac1 activity or ROS production in wild-type larvae by small molecule inhibitors was sufficient to induce hepatic steatosis. More conclusively, treating larvae with hydrogen peroxide, a diffusible ROS that has been implicated as a signaling molecule, alleviated hepatic steatosis in both GMP synthetase mutant and Rac1 inhibitor-treated larvae, indicating that homeostatic production of ROS is required to prevent hepatic steatosis. We further found that ROS positively regulate the expression of the triglyceride hydrolase gene, which is responsible for the mobilization of stored triglycerides in hepatocytes. Consistently, inhibition of triglyceride hydrolase activity in wild-type larvae by a small molecule inhibitor was sufficient to induce hepatic steatosis. Conclusion: de novo GMP synthesis influences the activation of the small GTPase Rac1, which controls hepatic lipid dynamics through ROS-mediated regulation of triglyceride hydrolase expression in hepatocytes.
Introduction
The earliest stage of nonalcoholic fatty liver disease (NAFLD), hepatic steatosis, is characterized by excess accumulation of triglycerides (TG) in hepatocytes as lipid droplets(1). Hepatic steatosis is a risk factor for progression to nonalcoholic steatohepatitis (NASH), which can result in end-stage liver disease(1). There have been no successfully established treatments for NAFLD or NASH, leaving the reduction of known risk factors as the standard of treatment. Thus, understanding the molecular mechanisms that underlie each stage of NAFLD pathogenesis could lead to the development of therapeutic targets to lessen or reverse NAFLD progression. A previous genome-wide association study in humans estimated the heritability of NAFLD to be 26–27%(2). However, the number of human genes known to associate with NAFLD is still limited(1), indicating the importance of finding new genes and pathways responsible for NAFLD pathogenesis.
In NAFLD pathogenesis, hepatic steatosis is induced by a net increase in the rate of TG acquisition and synthesis relative to export and oxidation. The removal of TGs from the liver is achieved by hydrolysis and subsequent β-oxidation of free fatty acids, or by secretion of lipoprotein particles containing TGs. Impairment of pathways regulating lipoprotein particle secretion can thus perturb the balance of TG homeostasis in the liver and lead to hepatic steatosis. Triglyceride hydrolase (TGH also known as Ces3 or Ces1d(3)) is an enzyme involved in the mobilization of stored TGs in hepatocytes to form lipoprotein particles(4–7).
In the progression of NAFLD from simple steatosis to NASH, it has been proposed that reactive oxygen species (ROS) play an important role(8). ROS are chemically reactive molecules containing oxygen, and common biological species include hydrogen peroxide (H2O2). ROS have been historically regarded as a toxic by-product of living cells that induce inflammatory responses and pathological conditions. Accumulating evidence now indicates, however, that ROS, especially the relatively stable H2O2 molecule, can function as intracellular second messengers at normal physiological levels(9, 10). The physiological role of ROS homeostasis in hepatocytes is, however, largely unknown.
In the cell, ROS can be generated in numerous biological reactions, primarily during mitochondrial metabolism and by ROS-generating enzymes, including the NOX family NADPH oxidases. The NADPH oxidases are multi-protein complexes that generate ROS(10). The roles of NADPH oxidases have been best characterized in phagocytes(10), however, this complex is found in many other tissues, including the liver. The regulatory subunit of Nox1 and Nox2 NADPH oxidases is the small GTPase Rac1(11), a member of the Rho GTPase family that regulates a wide variety of cellular functions. Rac1 works as a molecular switch by cycling between inactive and active states; when guanosine diphosphate (GDP) is bound, Rac1 is inactive, while Rac1 is activated by guanosine triphosphate (GTP) exchange. Therefore, GTP-bound Rac1 is necessary for the activation of Nox1 and Nox2 NAPDH oxidases.
GDP and GTP are generated from guanosine monophosphate (GMP) by transferring phosphate groups from ATP. In animal cells, GMP is synthesized through two distinct pathways: the de novo synthesis and salvage pathways(12). Since the salvage pathway is energetically more efficient, it is believed to be the primary supplier of guanine nucleotides. GTP is necessary for NOX2 NAPDH oxidase activation in vitro(13), but it is unclear how Rac1 and NADPH oxidase-mediated ROS generation is affected when guanosine nucleotides are reduced in vivo.
In this study, we implemented a forward genetic approach in zebrafish, which has proved to be a valuable strategy for identifying new genes and pathways that influence hepatic steatosis(14–18). We identified GMP synthetase mutant larvae as showing a hepatic steatosis phenotype, and subsequently found that they also show down-regulation of Rac1 activation and ROS generation. Accordingly, artificially reducing ROS levels through multiple mechanisms was sufficient to induce hepatic steatosis in wild-type zebrafish larvae, which were then subsequently rescued by artificially increasing ROS levels. These and other data suggest that physiological levels of ROS generation are required to protect the liver from accumulating excess hepatic lipid.
Materials and methods
Zebrafish husbandry and transgenic lines
Zebrafish (Danio rerio) larvae were obtained from crosses of wild-type AB/TL strain or heterozygous mutant fish and raised as previously described(19). The following transgenic and mutant lines were used: GMP synthetases850, Tg(fabp10:GFP-CAAX)lri1, and, Tg(fabp10:GFP-DNRac1)lri4.
Pharmacological treatments
The following molecules were used: Mycophenolic acid (Sigma Aldrich, Product #5255), Rac1 inhibitor (EMD Biosciences, Product #553502), diphenyleneiodonium chloride (DPI, Sigma Aldrich, Product #D2926), dimethyl p-nitrophenylphosphate (E600, Sigma Aldrich, Product #PS613) and N-acetyl-L-cystein (NAC, Sigma Aldrich, Product #A9165). All pharmacological treatments were administered with 1% dimethyl sulfoxide (DMSO) by volume. Concentrations of molecules used in this study are listed in supplementary table 2.
Oil Red O staining
Embryos were fixed at 7dpf and treated as previously described(17).
Rac1 activity assay
Rac1 Activity Assay Kit (Millipore) was used. Embryos were lysed and incubated with PAK-1 PBD-bound beads. After washing, beads were loaded on SDS-PAGE gels and blotted with anti-Rac1 (BD Transduction Laboratories, Cat. 610650) and β-tubulin (Abcam, Cat. 75123) antibodies.
Transmission electron microscopy
Electron microscopy was performed as previously described(19).
Nile Red staining, imaging and quantification
Embryos were fixed at 7dpf in 4% PFA overnight. Livers were removed and soaked in Nile Red (500ng/ml) along with TO-PRO3 Iodide nuclear stain for two hours at room temperature. Livers were then washed three times with PBS, mounted in Vectashield mounting media (Vector Laboratories) and imaged with a Leica SP5 confocal microscope (Leica Microsystems). Nile Red and GFP were simultaneously excited using a combination of 488 and 514 nm green argon lasers with emission of 505–520nm and 570–600nm, respectively. Images were then processed for 3-dimensional rendering using Imaris software (Bitplane Scientific).
The portion of hepatocytes containing lipid droplets was determined by blindly selecting a single z-plane from each confocal z-series and counting the number of cells that were positive or negative for the presence of lipid. Hepatocytes were identified by the expression of Tg(fabp10:GFP-CAAX)lri1.
Immunohistochemistry and in situ hybridization
Immunohistochemistry and in situ hybridization were performed as previously described(19).
Real-time reverse transcriptase-PCR
Quantitative RT-PCR was performed as previously described(19) using the primers listed in supplementary table 1. The ΔΔCt method was used for relative quantification.
Triglyceride measurement
Triglycerides were measured in whole-body extracts of larvae. Total lipids were quantified using the Triglyceride (GPO) Liquid Reagent assay kit (Pointe Scientific). Lipid concentration (mg/ml) was normalized to protein concentration (mg/ml) using the BCA Protein Assay Kit (Pierce, Rockford, IL) according to the manufacturers instructions.
ROS measurement
Following pharmacological treatments, live larvae were incubated in 30μM 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (H2DCF) for 90 minutes and culture media was then measured for fluorescence at 485/535 nanometers on a BMG Labtech Fluorostar Optima Fluorescent Plate Reader (Life Technologies). Background fluorescence was subtracted by measuring fluorescence in identical conditions containing no larva.
Hydrolase activity assay
12–15 larvae were collected following pharmacological treatment and lysed by sonication in 500μl PBS with 0.1% Triton X-100. Lysates were kept on ice and mixed 2:1 with the following solution: 180μg/ml SDS, 1% Triton, 350μg/ml p-nitrophenyl laurate (PNL) in PBS. PNL required incubation at 65°C for 20 minutes to solubilize, and was cooled to room temperature before use. Absorbance (405nm) was measured as Time30min – Time0min.
Statistics
All statistical tests were performed by unpaired, two-tailed t-test.
Results
Mutation in the GMP synthetase gene causes hepatic steatosis in zebrafish
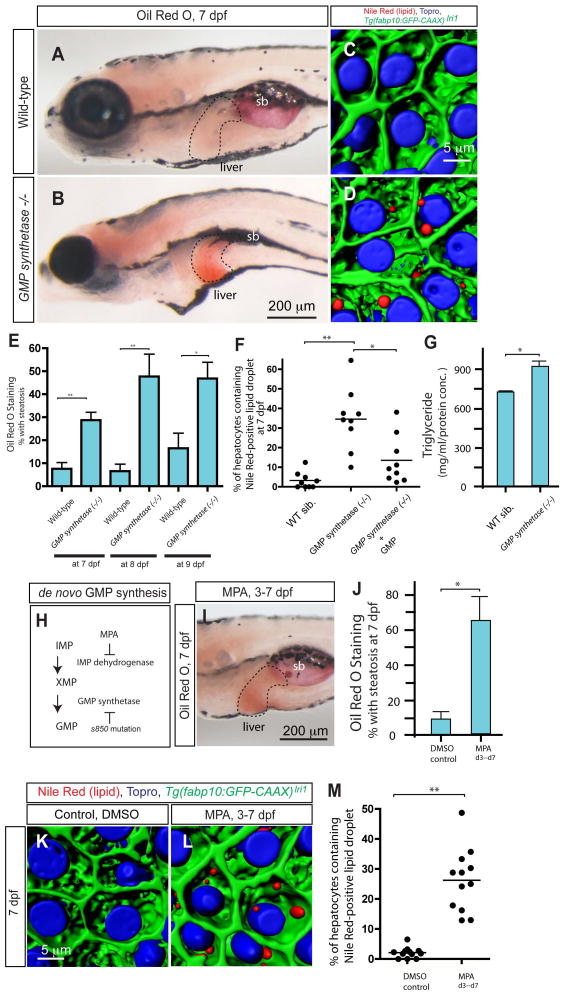
The s850 mutant was originally identified in a large-scale mutagenesis screen focusing on liver development(20) as a mutant showing reduced liver size (Supplementary fig. 1). A positional cloning approach identified the s850 mutation as a T to A transition in an exon of the GMP synthetase gene that changes the conserved histidine (H189) to glutamine (Q) (Supplementary fig. 2). Subsequently, we found that in GMP synthetases850 mutant larvae, hepatocytes start accumulating neutral lipid as indicated by whole-mount Oil Red O (ORO) staining at 7 days post fertilization (dpf) (Fig. 1A and B). Approximately 30% of GMP synthetases850 mutant larvae showed clear ORO staining in the liver at 7dpf (Fig. 1E). In GMP synthetases850 mutant larvae, ORO staining in the liver was also observed at 8 and 9 dpf (Fig. 1E). In order to investigate hepatic steatosis in GMP synthetases850 mutant larvae with subcellular-resolution, we developed a method in which we stained cytoplasmic lipid droplets by fluorescent Nile Red(21) and observed their intracellular localization in three dimensions by confocal microscopy (Fig. 1C and D). This method allowed us to precisely count the portion of hepatocytes containing one or more lipid droplets. In GMP synthetases850 mutant larvae, on average, 36.5% of hepatocytes contained Nile Red positive lipid droplets (S.D. 13.8; n=10; p < 0.01), while in their wild-type siblings, the percentage of hepatocytes containing Nile Red signal was significantly lower (average 5.5%; S.D. 6.7; n=9) (Fig. 1F). Consistently, when we treated GMP synthetases850 mutant larvae with 150 μM GMP, the percentage of hepatocytes containing lipid droplets was reduced (average 13.5%; S.D. 12.7; n=9), suggesting that insufficient GMP production might induce hepatic steatosis in GMP synthetases850 mutant larvae. Electron micrographs confirmed the existence of lipid droplets in GMP synthetases850 mutant hepatocytes (Supplementary fig. 3). Consistent with hepatic steatosis, the total triglyceride level is increased in GMP synthetases850 mutant larvae (Fig. 1G).
Figure 1.
Lack of de novo GMP synthesis leads to hepatic steatosis. (A and B) Lateral views of wild-type sibling and GMP synthetases850 mutant larvae stained with Oil Red O (ORO) at 7 days post fertilization (dpf). GMP synthetases850 mutant larvae develop liver steatosis at 7 dpf. Broken lines outline the liver. (C and D) Projected confocal images of the wild-type sibling (C) and GMP synthetases850 mutant (D) liver visualized for Nile Red (red) and To-pro-3 (blue) staining and Tg(fabp10:GFP-CAAX)lri1 (green) expression at 7 dpf. In GMP synthetases850 mutant larvae, lipid droplets stained by Nile Red are evident in hepatocytes. (E) The percentage of wild-type and GMP synthetases850 mutant larvae showing liver steatosis scored by whole-mount ORO staining at 7, 8 and 9 dpf. ORO staining experiments with wild-type larvae at 7, 8 and 9 dpf were repeated five times (total n = 138 larvae examined and total n = 4 showed ORO signal in the liver), five times (total n = 74 larvae examined and total n = 9 showed ORO signal in the liver) and six times (total n = 67 larvae examined and total n = 13 showed ORO signal in the liver), respectively. ORO staining experiments with GMP synthetases850 mutant larvae at 7, 8 and 9 dpf were repeated five times (total n = 124 larvae examined and total n = 36 showed ORO signal in the liver), four times (total n = 34 larvae examined and total n = 15 showed ORO signal in the liver) and three times (total n = 26 larvae examined and total n = 12 showed ORO signal in the liver), respectively. (F) Quantification of liver steatosis measured by the percentage of hepatocytes containing Nile Red positive lipid droplets in wild-type sibling (n = 9), GMP synthetases850 mutant (n = 9) and 150μM GMP treated GMP synthetases850 mutant larvae (n = 9). The percentage of hepatocytes containing lipid droplets is significantly increased in GMP synthetases850 mutant larvae. (G) Triglyceride levels measured in whole-body extracts of wild-type and GMP synthetases850 mutant larvae at 7 dpf. (H) A schematic of the de novo GMP synthesis pathway. In the linear de novo GMP synthesis pathway, IMP is converted to XMP by IMP dehydrogenase, and subsequently, XMP is converted to GMP by GMP synthetase. Mycophenolic acid (MPA) inhibits the function of IMP dehydrogenase. The s850 mutation disrupts the function of GMP synthetase. (I) Wild-type larvae exposed to MPA from 3 to 7 dpf were fixed and stained with ORO at 7 dpf. MPA-treated larvae developed liver steatosis. (J) The percentage of control DMSO or MPA-treated larvae showing liver steatosis scored by whole-mount ORO staining at 7dpf. MPA treatment increased the percentage of larvae showing liver steatosis. ORO staining experiments with MPA-treated larvae were repeated three times with an average n = 35.4 larvae per experiment (total n = 106 larvae examined and total n = 70 larvae showed ORO signal in the liver). (K and L) Projected confocal images of wild-type larvae treated with DMSO (K) or MPA (L) from 3 to 7 dpf visualized for Nile Red (Red) and To-pro-3 (Blue) staining and Tg(fabp10:GFP-CAAX)lri1 expression (green). Nile Red stains lipid droplets, and Topro stains nuclei. (M) Quantification of liver steatosis measured by the percentage of hepatocytes containing lipid droplets in DMSO-treated control and MPA-treated larvae at 7 dpf. MPA-treatment significantly increased the percentage of hepatocytes containing lipid droplets. sb, swim bladder. *P<0.05, **P<0.01; error bars indicate standard deviation.
De novo GMP synthesis is required to prevent hepatic steatosis
Previous studies indicated that the proliferation of leukocytes in mammals(22) and axon guidance in the Drosophila visual system(23) require de novo GMP synthesis. However, the precise mechanisms by which de novo GMP synthesis regulates these biological processes are not clear. The final two steps of the de novo GMP synthesis pathway are linear and rate-limiting steps in which inosine monophosphate (IMP) dehydrogenase catalyzes the oxidation of IMP to xanthosine monophosphate (XMP) and GMP synthetase catalyzes the amination of XMP to GMP (Fig. 1H). In order to distinguish whether de novo GMP synthesis or unknown signaling or regulatory effects of GMP synthetase are responsible for hepatic steatosis, we treated wild-type zebrafish larvae with mycophenolic acid (MPA), a small molecule inhibitor of IMP dehydrogenase(24), to downregulate de novo GMP synthesis activity. We found that treating wild-type larvae with 15 μg/ml MPA from 3 to 7 dpf induced hepatic steatosis at 7 dpf (Fig. 1I and J), suggesting inhibition of de novo GMP synthesis is sufficient to induce hepatic steatosis. Consistently, we counted the number of Nile Red-positive hepatocytes in MPA-treated larvae (Average 26.2%; S.D. 10.5; n=12) and found significantly more hepatocytes containing lipid droplets (Fig. 1K-M) than in control DMSO-treated larva (average 2.1%; S.D. 1.7; n=12). Altogether, these data uncover a new role for de novo GMP synthesis in TG metabolism and hepatic steatosis in zebrafish.
De novo GMP synthesis is required for activation of the small GTPase Rac1
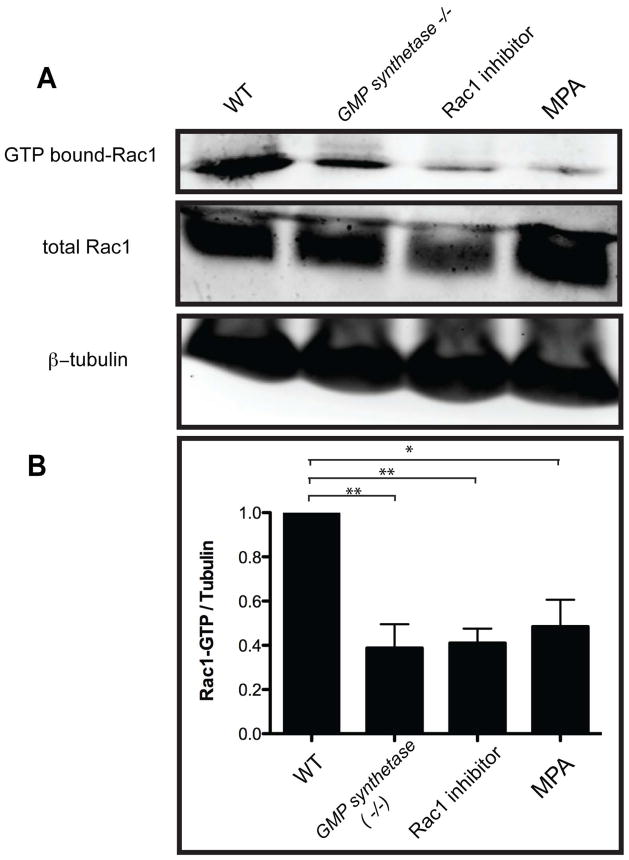
In Drosophila, de novo GMP synthesis is required to activate the small GTPase Rac1(23). We hypothesized that in zebrafish GMP synthetases850 mutant larvae, the supply of GMP, and hence GTP, is compromised, leading to a decrease in the activity of small GTPases including Rac1. To test this hypothesis, we measured GTP-bound (activated) Rac1 levels using a Pak1-binding domain (PBD) pull-down assay (Fig. 2A). We found that GTP-bound Rac1 levels are decreased in GMP synthetases850 mutant and MPA-treated larvae (Fig. 2), suggesting that de novo GMP synthesis is required for the full activation of Rac1.
Figure 2.
De novo GMP synthesis is required for the activation of Rac1. (A) Guanosine 5′-triphosphate (GTP)-bound Rac1 levels in wild-type, GMP synthetases850 mutant, Rac1 inhibitor treated and MPA treated larvae at 7 dpf were measured by using the PBD pull-down assay. (B) Densitometric analysis of GTP-bound Rac1 levels from three independent experiments. GTP-bound Rac1 levels were determined by using the PBD pull-down assay and normalized to β-tubulin levels. All tested conditions showed significant down-regulation of activated Rac1. *P<0.05, **P<0.01; error bars indicate standard deviation.
Inhibition of Rac1 activity in hepatocytes induces hepatic steatosis
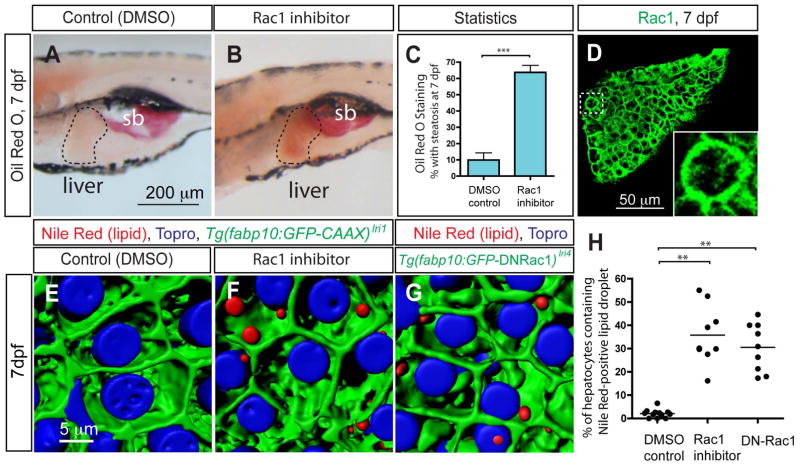
Interestingly, we found that inhibiting Rac1 activity is sufficient to induce hepatic steatosis (Fig. 3A and B). When treated with 50 μg/ml Rac1 inhibitor-containing media for 48 hours from 5 dpf, the activity of Rac1 was down-regulated in larvae (Fig. 2) as expected, and we found that a majority of treated larvae developed hepatic steatosis as indicated by increased Oil Red O staining in liver (Fig. 3B and C). To our knowledge, this is the first in vivo data suggesting a link between small GTPases and the regulation of hepatic steatosis. We counted the number of Nile Red-positive hepatocytes in Rac1 inhibitor-treated larvae (average 35.6%; S.D. 12.5; n=9) and found significantly more hepatocytes containing lipid droplets than in DMSO-treated control larvae (average 2.1%; S.D. 1.7; n=12) (Fig. 3E, F and H).
Figure 3.
The small GTPase Rac1 regulates hepatic steatosis. (A and B) Lateral views of wild-type larvae treated with DMSO (A) or Rac1 inhibitor (B) from 5 to 7 dpf and stained for ORO at 7 dpf. Rac1 inhibitor-treated larvae developed hepatic steatosis. (C) The percentage of DMSO or Rac1 inhibitor-treated larvae showing liver steatosis scored by whole-mount ORO staining. ORO staining experiments with Rac1 inhibitor-treated larvae were repeated twelve times with an average n = 12.1 larvae per experiment (total n = 146 larvae examined and total n = 89 larvae showed ORO signal in the liver). (D) Z-plane confocal image of the dissected liver of wild-type larvae visualized for Rac1 expression at 7 dpf. Rac1 is localized to the entire cell membrane of most cells in the liver. The outlined area is magnified and shown in bottom right corner. (E and F) Projected confocal images of lipid droplets in the liver stained by Nile Red. Tg(fabp10:GFP-CAAX)lri1 larvae treated with DMSO (E) or Rac1 inhibitor (F) visualized for GFP expression and Nile Red (Red) and To-pro-3 (Blue) staining at 7dpf. (G) Projected confocal image of Tg(fabp10:GFP-DNRac1)lri4 liver visualized for GFP expression and Nile Red (Red) and To-pro-3 (Blue) staining at 7dpf. (H) Quantification of liver steatosis measured by the percentage of hepatocytes containing Nile Red positive lipid droplets in DMSO-treated control, Rac1 inhibitor-treated and Tg(fabp10:GFP-DNRac1)lri4 larvae at 7 dpf. *P<0.05, **P<0.01, ***P<0.001; error bars indicate standard deviation.
After observing that Rac1 is expressed strongly in hepatocytes at 7 dpf (Fig. 3D; supplementary fig. 4), we hypothesized that Rac1 activity in hepatocytes is required for the prevention of hepatic steatosis. To test this hypothesis, we generated a new transgenic line, Tg(fabp10:GFP-DNRac1)lri4, which expresses dominant negative Rac1 (N17) only in hepatocytes (Supplementary fig. 5). In Tg(fabp10:GFP-DNRac1)lri4 larvae, the percentage of hepatocytes containing lipid droplets stained by Nile Red is significantly higher (average 32.7%; S.D. 11.9; n=12) (Fig. 3G and H; supplementary fig. 5), suggesting that Rac1 activity in hepatocytes is important for the regulation of hepatic steatosis.
Homeostatic generation of ROS is necessary to prevent hepatic steatosis
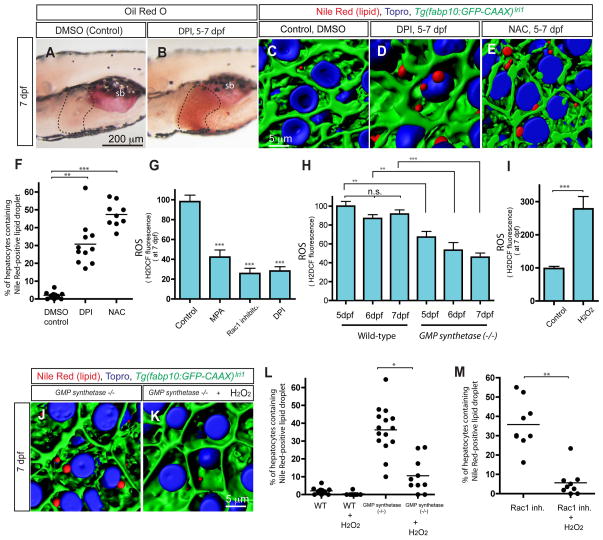
Historically, the role of Rac1 in actin cytoskeletal reorganization has been extensively studied(25); however, it is also known that Rac1 forms a protein complex with NADPH oxidases (Nox) to regulate their function in generating the superoxide anion that is quickly dismuted to hydrogen peroxide (H2O2) and other ROS molecules(10, 11, 26). Since accumulating evidence indicates that ROS are important components in cell signaling, we hypothesized that Rac1 regulates hepatic steatosis through Nox-mediated ROS production. To test this hypothesis, we inhibited the activity of Nox by the flavoprotein inhibitor, diphenyliodonium(10) (DPI). We found that larvae treated with 10 μM DPI from 5 dpf showed strong Oil Red O signal in the liver at 7 dpf (Fig. 4A and B). We also confirmed that the percentage of hepatocytes containing lipid droplets stained by Nile Red is significantly higher in DPI-treated larva (Average 30.8%; S.D. 12.5; n=11) (Fig. 4D and F). These data suggest that down-regulating Nox activity is sufficient to induce hepatic steatosis. To test whether Nox mediated ROS production is important for the prevention of hepatic steatosis, we treated larvae with the ROS-quenching agent N-acetyl cysteine(8) (NAC). We found that in larvae treated with 50 μM NAC from 5 dpf, the percentage of hepatocytes containing lipid droplets is significantly increased at 7 dpf (average 47.4%; S.D. 6.9; n=9) (Fig. 4E and F). These data suggest that a reduction in ROS production might be responsible for the induction of hepatic steatosis.
Figure 4.
Reactive Oxygen Species (ROS) homeostasis is necessary for the prevention of hepatic steatosis. (A and B) Lateral views of wild-type larvae treated with DMSO (A) or diphenyleneiodonium chloride (DPI) (B) from 5 to 7 dpf and stained for ORO at 7 dpf. Flavoprotein inhibitor, DPI, treated larvae (average 67.2%; s.d. 12.6; p<0.001) developed hepatic steatosis. ORO staining experiments with DPI-treated larvae were repeated five times with an average n = 12.2 larvae per experiment (total n = 61 larvae examined and total n = 41 larvae showed ORO signal in the liver). (C–E) Projected confocal images of lipid droplets in the liver stained by Nile Red. Tg(fabp10:GFP-CAAX)lri1 larvae treated with DMSO (C), DPI (D) or N-acetyl cysteine (NAC) (E) visualized for GFP expression and Nile Red (Red) and To-pro-3 (Blue) staining at 7dpf. (F) Quantification of liver steatosis measured by the percentage of Nile Red positive lipid droplets containing hepatocytes in DMSO, DPI or NAC treated larvae at 7 dpf. (G–I) Measurement of whole-body ROS production by H2DCF fluorescence. Arbitrary units of H2DCF fluorescence from three different experiments (n > 50) were normalized to control and averaged. Production of ROS in control, MPA-treated, Rac1 inhibitor-treated, and DPI-treated larvae was measured at 7 dpf (G). All tested conditions showed significant reduction of ROS production in (G). Production of ROS in wild-type and GMP synthetases850 mutant larvae was measured at 5, 6 and 7 dpf (H). In wild-type larvae, the ROS production levels between 5 and 7 dpf were not significantly changed, while in GMP synthetases850 mutant larvae, the ROS production level at 7dpf was reduced compared to that at 5 dpf (H). Production of ROS in control and hydrogen peroxide-treated larvae was measured at 7 dpf (I). (J and K) Hepatic steatosis in GMP synthetases850 mutant larvae was ameliorated by hydrogen peroxide treatment. Projected confocal images of lipid droplets in the liver stained by Nile Red. GMP synthetases850 mutant larvae cultured in the absence (J) or presence (K) of 1mM hydrogen peroxide were visualized for for Nile Red (red) and To-pro-3 (blue) staining and Tg(fabp10:GFP-CAAX)lri1 expression (green) at 7 dpf. Nile Red positive lipid droplets in the liver are reduced in hydrogen peroxide-treated GMP synthetases850 mutant larvae. (L) Quantification of liver steatosis measured by the percentage of hepatocytes containing Nile Red positive lipid droplet in wild-type, hydrogen peroxide-treated wild-type, GMP synthetases850 mutant and hydrogen peroxide-treated GMP synthetases850 mutant larvae at 7 dpf. Hydrogen peroxide treatment ameliorated hepatic steatosis in GMP synthetases850 mutant larvae. (M) Quantification of liver steatosis measured by the percentage of hepatocytes containing Nile Red positive lipid droplets in Rac1 inhibitor-treated and Rac1 inhibitor plus hydrogen peroxide-treated larvae at 7dpf. Hydrogen peroxide treatment suppressed Rac1 inhibitor induced hepatic steatosis. n.s., not significant; *P<0.05, **P<0.01, ***P<0.001; error bars indicate standard deviation.
To test this hypothesis, we next measured whole-body ROS production in DPI-treated, MPA-treated, Rac1 inhibitor-treated and GMP synthetases850 mutant larvae. As expected, in 10 μM DPI-treated larva, the production of ROS throughout the body was significantly reduced (Fig. 4G). Indeed, we found that ROS production was also reduced in MPA-treated and Rac1 inhibitor-treated and GMP synthetases850 mutant larvae (Fig. 4G and H), supporting the hypothesis that a reduction in ROS production might be responsible for the induction of hepatic steatosis. To further test this hypothesis, we treated larvae with 1mM hydrogen peroxide (H2O2), which increased internal ROS levels as indicated by fluorescence of the ROS indicator, H2DCF (Fig. 4I) without any morphological changes at 7dpf, and asked if increasing ROS levels would rescue hepatic steatosis. When we treated GMP synthetases850 mutant larvae with 1mM H2O2 from 4 to 7 dpf, lipid droplets in hepatocytes were significantly decreased (average 10.5%; S.D. 9.7; n=10; p<0.05) (Fig. 4J–L), suggesting that artificially increasing ROS ameliorated hepatic steatosis in GMP synthetase mutant larvae. Consistently, 1mM H2O2 treatment from 5 to 7 dpf, eliminated lipid droplets in hepatocytes of Rac1 inhibitor-treated larvae (average 1.8%; S.D. 2.9; n=9; p<0.01) (Fig. 4M), further supporting the notion that ROS homeostasis is important for the prevention of hepatic steatosis.
ROS production influences triglyceride hydrolase gene expression
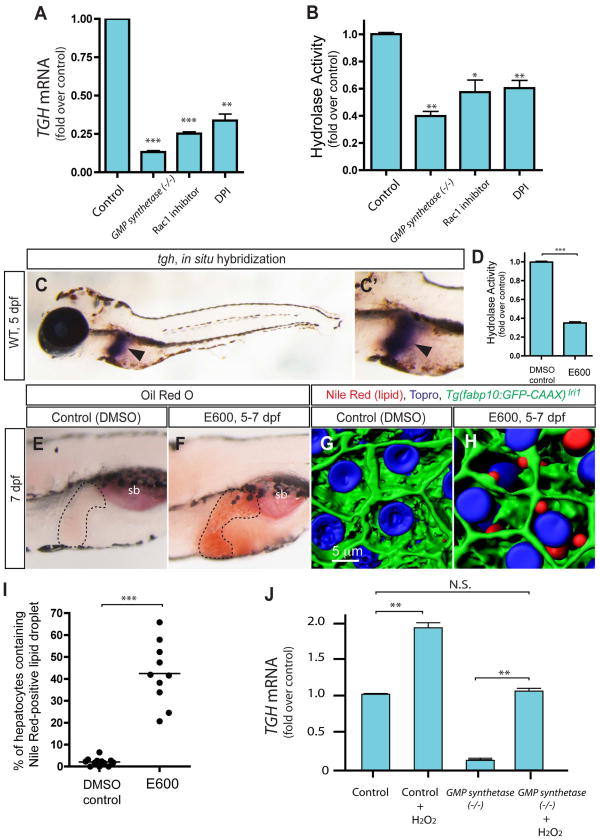
To understand the molecular mechanisms by which ROS generation influences hepatic steatosis, we used microarray analysis to look for genes that are affected by both the GMP synthetase mutation and Rac1 inhibitor-treatment in a similar manner. Among the candidates satisfying this criterion, we focused on the triglyceride hydrolase (tgh) gene, which codes for an enzyme responsible for the mobilization of stored triglyceride in hepatocytes(4, 5, 7). We first verified the down-regulation of tgh gene expression in GMP synthetases850 mutant and Rac1 inhibitor-treated larvae by quantitative RT-PCR (qPCR, Fig. 5A). Since down-regulation of Triglyceride hydrolase (TGH) activity is sufficient to induce lipid droplet accumulation in hepatocytes(4), we hypothesized that the Rac1 mediated ROS production regulates tgh gene expression to control lipid droplet formation in hepatocytes. Supporting this hypothesis, it was found that the expression level of the tgh gene was also reduced in DPI-treated larvae (Fig. 5A). Consistent with these gene expression changes, the enzymatic activity of TGH(6) in GMP synthetases850 mutant, Rac1 inhibitor-treated, and DPI-treated larvae was also reduced (Fig. 5B), suggesting that Rac1 mediated ROS production influences TGH activity by regulating its expression. The tgh gene is expressed only in the yolk syncytial layer (YSL) at 3 dpf (data not shown), however its expression is restricted to the liver by 5 dpf and it remains expressed only in the liver at 7dpf (Fig. 5C).
Figure 5.
Expression of the triglyceride hydrolase (tgh) gene is regulated by Rac1 mediated ROS. (A) qPCR analysis of tgh mRNA expression levels in wild-type control, GMP synthetases850 mutant, Rac1 inhibitor-treated and DPI-treated larvae at 7 dpf. The averages of at least three independent experiments are shown. tgh mRNA expression level is significantly down-regulated in all tested conditions. (B) Hydrolysis activity toward p-nitrophenyl laurate was measured using lysates of control, GMP synthetases850 mutant, Rac1 inhibitor-treated and DPI-treated larvae at 7 dpf. Measured activity was normalized to control and the average of three different experiments is shown. Hydrolysis activities are significantly decreased in all tested cases. (C) tgh expression in 5 dpf wild-type larvae. Lateral views, anterior to the left. The region around the liver is magnified and shown separately in C′. The expression of tgh is restricted to the liver at this stage. Arrowheads point to the liver. (D) The hydrolysis activity of the homogenate of 7dpf larvae is measured in the absence or presence of Dimethyl-p-nitrophenyl phosphate (E600). E600 significantly down-reregulated hydrolysis activity. (E and F) Lateral views of DMSO- (C) or E600- (D) treated larvae stained for ORO at 7 dpf. Hydrolase inhibitor, E600, treated larvae (Average 62.2%; s.d. 10.6; p<0.001) developed hepatic steatosis. The black broken lines outline the liver. ORO staining experiments with E600-treated larvae were repeated six times with an average n = 14 larvae per experiment (total n = 84 larvae examined and total n = 52 larvae showed ORO signal in the liver). (G and H) Projected confocal images of lipid droplets in the liver stained by Nile Red. Tg(fabp10:GFP-CAAX)lri1 larvae treated with DMSO (G) or E600 (H) visualized for GFP expression and Nile Red (Red) and To-pro-3 (Blue) staining at 7dpf. (I) Quantification of liver steatosis measured by the percentage of hepatocytes containing Nile Red positive lipid droplets in DMSO- or E600-treated larvae at 7 dpf. E600-treated larvae developed hepatic steatosis. (J) qPCR analysis of tgh mRNA expression levels in wild-type, hydrogen peroxide-treated wild-type, GMP synthetases850 mutant and hydrogen peroxide-treated GMP synthetases850 mutant larvae. tgh mRNA expression levels were restored to the level of wild-type control in hydrogen peroxide treated GMP synthetases850 mutant larvae. *P<0.05, **P<0.01, ***P<0.001, n.s., not significant; error bars indicate standard deviation.
Inhibition of hydrolase activity in cultured hepatocytes by a small molecule inhibitor, Diethyl p-nitrophenyl phosphate (E600), was shown to attenuate mobilization of TG7(7). Thus, we hypothesized that decreased hydrolase activity causes hepatic steatosis by attenuating the mobilization of transiently stored TG in hepatocytes. To test this hypothesis, we treated zebrafish larvae with 10 μM E600 from 5 to 7 dpf and found that the hydrolase enzymatic activity was reduced in E600-treated larvae (Fig. 5D). We further found that E600-treated larvae developed strong hepatic steatosis (Fig. 5F and H), with more than 20% of hepatocytes containing lipid droplets stained by Nile Red (Fig. 5G–I) in all E600-treated larvae examined (average 42.4%; S.D. 14.1; n=10), suggesting that the inhibition of hydrolase activity is sufficient to induce hepatic steatosis in zebrafish larvae.
Since decreased ROS production down-regulates tgh gene expression, we hypothesized that ROS production levels are correlated with tgh gene expression levels. To test this hypothesis, we treated larvae with 1mM H2O2 from 5 to 7 dpf and examined the expression level of tgh mRNA. We found that tgh mRNA expression was increased in the 1mM H2O2 -treated larvae (Fig. 5J), supporting the hypothesis that ROS levels positively regulate tgh gene expression. Finally, we treated GMP synthetases850 mutant larvae, in which ROS production and tgh gene expression are reduced, with 1mM H2O2 from 5 to 7 dpf, and examined the expression level of the tgh mRNA. We found that tgh mRNA expression was restored to wild-type levels in H2O2-treated GMP synthetases850 mutant larva (Fig 5J), suggesting that increasing ROS is sufficient to rescue down-regulation of tgh transcription in GMP synthetases850 mutant larvae.
Discussion
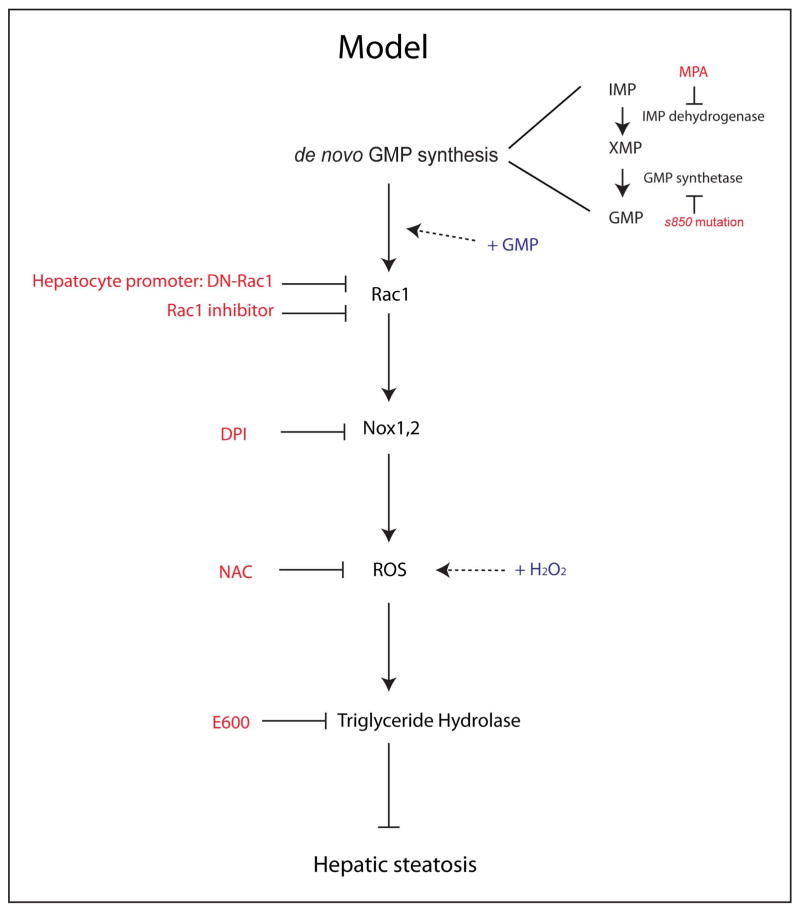
In this study, we show that de novo GMP synthesis is necessary to prevent hepatic steatosis in zebrafish (Fig. 1). De novo GMP synthesis influences the activation of the small GTPase Rac1, and Rac1 promotes the production of reactive oxygen species (ROS) (Fig. 6), which is important in regulating hepatic lipid dynamics by controlling triglyceride hydrolase gene expression (Fig. 6). Given that down-regulating Rac1 activity, by overexpressing dominant negative Rac1 specifically in hepatocytes, was sufficient to induce hepatic steatosis (Fig. 3G and H), this signaling cascade appears to take place in hepatocytes.
Figure 6.
Schematic model of ROS mediated liver protection from hepatic steatosis. Inhibiting de novo GMP synthesis by MPA-treatment or the GMP synthetases850 mutation induced hepatic steatosis. Supplying GMP rescued the hepatic steatosis phenotype in GMP synthetases850 mutant larva. De novo GMP synthesis influenced the activation of Rac1 and inhibiting Rac1 activity in hepatocytes by overexpressing dominant negative Rac1 (DN-Rac1) also induced hepatic steatosis. Rac1 regulates the activity of NADPH oxidases (Nox1, 2) and inhibiting NADPH oxidases mediated ROS production by DPI or quenching ROS by N-acetyl cysteine (NAC) also induced hepatic steatosis. Supplying hydrogen peroxide (H2O2) rescued the hepatic steatosis phenotype in GMP synthetases850 mutant, Rac1 inhibitor-treated, and DN-Rac1 expressing larvae. ROS levels influenced triglyceride hydrolase gene expression. The small molecule inhibitor for Triglyceride hydrolase (E600) treatment also induced hepatic steatosis.
Mycophenolic acid influences the production of ROS
Hydrogen peroxide (H2O2), a relatively stable ROS that functions as a signaling molecule, mediates communication between wounded tissues and leukocytes(27). Mycophenolic acid (MPA) is a Food and Drug Administration (FDA) approved drug that has been used for immunosuppression(24), however the precise molecular mechanisms by which MPA suppresses immune reaction are not clear. In this study, we show that in MPA-treated larvae, the production of ROS was reduced (Fig. 4F). This reduction in ROS production is most likely due to reduced activation of Rac1 (Fig. 2). Based on these results, we suggest that MPA suppresses the immune response, at least in part, by affecting Rac1 mediated ROS production.
Hepatic steatosis in GMP synthetase mutant larvae
We observed hepatic steatosis in GMP synthetases850 mutant larvae (Fig. 1). Consistently, we also observed increased total triglyceride (TG) level in these animals (Fig. 1G), however, since we measured the triglyceride level in a whole-body, this could be due to increased TG levels in extrahepatic tissues.
Although both intrahepatic biliary and vascular networks exist in GMP synthetases850 mutant larvae at 7 dpf (Supplementary fig 6), their livers are smaller likely due to reduced cell proliferation (Supplementary fig. 1). Since liver size is not rescued in hydrogen peroxide (H2O2)-treated GMP synthetases850 mutant larvae (data not shown), and Rac1 inhibitor-treated, DPI-treated, E600-treated (data not shown) and Tg(fabp10:GFP-DNRac1)lri4 larvae have normal liver size (Supplementary fig. 5), we conclude that the liver cell proliferation phenotype in GMP synthetases850 mutant larvae appears to be independent of the ROS-mediated pathway.
Consistent with a previous study(28), GMP synthetases850 mutant larvae also display smaller eyes, the absence of xanthopore pigmentation, and dysmorphic branchial arches. However, these phenotypes were not rescued by H2O2 treatment (data not shown), suggesting that these phenotypes are also independent of the ROS-mediated pathway.
Hepatic steatosis is a risk factor for progression to nonalcoholic steatohepatitis (NASH), which is associated with inflammation. In GMP synthetases850 mutant larvae, inflammation is not evident at 7 dpf, as evidenced by a lack of neutrophil infiltration to the liver at this stage (Supplementary fig. 7). However, these data do not exclude the possibility of the presence of other types of immune cells in the livers of GMP synthetases850 mutant larvae.
At 7 dpf, the percentage of GMP synthetases850 mutant larvae showing ORO staining in the liver is relatively low (Fig. 1E). Since MPA treatment to GMP synthetases850 mutant larvae further increased the percentage of ORO staining at 7 dpf (Supplementary fig. 8), maternally deposited GMP synthetase mRNA or protein might be influencing the results, or the s850 allele might not be a null.
We did not observe any hepatic steatosis at 5 or 6 dpf in GMP synthetases850 mutant larvae (data not shown). Similarly, Rac1 inhibitor or DPI treatment from 3 to 5 dpf did not induce hepatic steatosis in wild-type larvae (Supplementary fig. 9). The observation that the triglyceride hydrolase (tgh) gene is expressed in the liver only after 5 dpf (Fig. 5C) may explain why down-regulating ROS production does not induce hepatic steatosis before 5 dpf. Consistent with this hypothesis, Rac1 inhibitor or DPI treatment induces significant hepatic steatosis after 6 dpf both in starved and fed wild-type larvae (Supplementary Fig. 9 and 10).
Triglyceride hydrolase (tgh) gene regulation by ROS levels
We showed that expression of tgh is correlated with ROS levels. In GMP synthetases850 mutant, Rac1 inhibitor-treated, and DPI-treated larvae, in which ROS production is reduced (Fig. 4G and H), relative tgh expression is down-regulated (Fig. 5A). Conversely, in H2O2-treated larvae, the expression of tgh was increased (Fig. 5J). Down-regulating TGH activity level by E600 (Fig. 5D) to the level in GMP synthetases850 mutant larvae (Fig. 5B) was sufficient to induce hepatic steatosis (Fig. 5E–I), supporting the hypothesis that reduced tgh expression is responsible for hepatic steatosis in GMP synthetases850 mutant larvae.
A previous study indicated that hepatic TG levels in Tgh-null mice were not statistically different from those in wild-type mice(5). In mice, Tgh also acts in white adipose tissues(5) and it is likely that the absence of hepatic steatosis in tgh-null mice is due to decreased fatty acids delivery to the liver from adipose tissue, since isolated Tgh-null hepatocytes in culture accumulate more exogenous lipids than wild-type hepatocytes(4). In contrast, zebrafish white adipose tissues only develop after 12 dpf(29), potentially explaining why suppressing Tgh activity was sufficient to induced hepatic steatosis at 7 dpf.
In GMP synthetases850 mutant larvae, expression of genes involved in de novo lipogenesis (srebp1, acc1, agapt, and fads2), β-oxidation (aco, cpt1, cyp4a10, and echs1) or lipid uptake (cd36) are not significantly changed at 6 dpf (Supplementary fig. 11). These data also support the hypothesis that reduced tgh expression is responsible for hepatic steatosis in GMP synthetases850 mutant larvae.
Under physiological conditions, ROS produced by β-oxidation of triglyceride-derived free fatty acids may provide feedback to influence Tgh activity, adjusting lipid dynamics in hepatocytes.
ROS and hepatic steatosis
ROS are recognized to play important roles in host defense, especially in the innate immune response of leukocytes to pathogens(10), though the excessive production of ROS frequently results in inflammatory responses in many tissues, including the liver. In the liver, the two hit model has been proposed for the transition of hepatic steatosis to more severe NASH, in which the first hit is hepatic steatosis and the second hit is ROS-mediated inflammation(30). Our data provide genetic evidence that physiological ROS levels are also necessary for the prevention of hepatic steatosis in zebrafish larvae (Fig. 6). The ability of H2O2 to rescue hepatic steatosis in GMP synthetases850 mutant, Rac1 inhibitor-treated, and Tg(fabp10:GFP-DNRac1)lri4 larvae (Fig. 4 and 6) further supports this idea. Our data do not, however, conflict with the current two hit model or the notion that excess ROS production is pathological; rather we propose that a reduction in physiological levels of ROS can be equally pathogenic to increased levels of ROS. These data suggest that proposed antioxidant supplementation for the treatment of NAFLD(31) would require careful dosage control to ensure that ROS levels are not reduced below their physiologically normal levels.
Supplementary Material
Supplementary Figure 1. Liver size is reduced in GMP synthetases850 mutant larvae. Brightfield images of wild-type (A) and GMP synthetases850 mutant (B) larvae at 7 dpf are overlaid with Tg(fabp10:GFP-CAAX)lri1 expression (green). GFP expression is shown separately in A′ and B′. (C) EdU incorporation rate in the liver of wild-type and GMP synthetases850 mutant larvae at 4 dpf. The cell proliferation rate, indicated by EdU incorporation, is lower in the liver of GMP synthetases850 mutant larvae at this stage. EdU incorporation was measured as previously described(s1).
Supplementary Figure 2. Positional cloning of the s850 locus. (A) Bulk segregant analyses using markers from the zebrafish genetic map placed the s850 locus on zebrafish chromosome 18 near z14694. We further defined a critical region surrounding the s850 locus, which was flanked by markers 0.05 cM (SCA-KB5) and 0.1 cM (SCA-GA4) from the s850 locus. (B) Sequencing genes in the critical region identified a single nucleotide change (T to A) in the GMP synthetase gene. (C) The s850 mutation changes T589 to A589 of the GMP synthetase gene. (D) The s850 mutation replaces histidine (H189) of GMP synthetase with glutamine (Q189).
Supplementary Figure 3. Electron micrographs of the GMP synthetases850 mutant liver. Electron micrographs of the wild-type (E) and GMP synthetases850 mutant liver at 7 dpf are shown. In GMP synthetases850 mutant larvae, lipid droplets are evident in hepatocytes. l,lipid droplet; n, nucleus.
Supplementary Figure 4. Rac1 expression in the liver. Z-plane confocal image of the dissected liver of wild-type larvae at 7 dpf. (A) Rac1 expression (green) and DAPI staining (white) are visualized. The green channel is shown separately in A′. (B) Mouse IgG1 (geen), used as a negative control in immunofluorescence, and DAPI staining (white) are visualized. The green channel is shown separately in B′. There was no signal detected with mouse IgG1.
Supplementary Figure 5. Tg(fabp10:GFP-DNRac1)lri4 larvae show no morphological defects. (A) Bright-field images of Tg(fabp10:GFP-DNRac1)lri4 larvae at 7 dpf are overlaid with Tg(fabp10:GFP-CAAX)lri1 expression (green). Tg(fabp10:GFP-DNRac1)lri4 larvae are viable and their physical appearance is normal. The liver size of Tg(fabp10:GFP-DNRac1)lri4 larvae is indistinguishable from that of wild-type larvae. (B) Quantification of liver steatosis measured by the percentage of hepatocytes containing Nile Red positive lipid droplets in wild-type sibling, Tg(fabp10:GFP-CAAX)lri1 and hydrogen peroxide-treated Tg(fabp10:GFP-CAAX)lri1 larvae. The percentage of hepatocytes containing lipid droplets is significantly increased in Tg(fabp10:GFP-CAAX)lri1 mutant larvae and is ameliorated by the hydrogen peroxide treatment.
Supplementary Figure 6. The intrahepatic biliary and vascular networks exist in GMP synthetases850 mutant larvae. Wild-type (A) and GMP synthetases850 mutant (B) Tg(Tp1-MmHbb:EGFP)um14 Tg(kdrl:memCherry)s896 larvae visualized for GFP, mCherry, and DAPI expression. Z-plane confocal images of the liver at 7 dpf. GFP and mCherry expressions are shown separately in (A′ and B′) and (A″ and B″), respectively. Ventral views, anterior to the top. Although the liver size is smaller, the intrahepatic biliary and vascular networks, visualized by Tg(Tp1-MmHbb:EGFP)um14 and Tg(kdrl:memCherry)s896 expressions(s2, s3) respectively, appear to be formed in GMP synthetases850 mutant larvae.
Supplementary Figure 7. Neutrophil infiltration to the liver is not observed in GMP synthetases850 mutant larvae. (A–F) Myeloid peroxidase (MPO) staining visualizing neutrophils. Wild-type (A and D), GMP synthetases850 mutant (B and E), and 150 μg/ml lipopolysaccharide (LPS) treated wild-type larvae at 7dpf with MPO-positive neutrophils visualized in dark brown in the livers (A–C) and posterior cardinal vein (D–F; arrows). There were no MPO-positive cells observed in the liver of wild-type and GMP synthetases850 mutant larvae, while MPO-positive cells do infiltrate the liver of LPS-treated larvae (arrowheads in C). These data suggest that inflammation, indicated by neutrophil infiltration, is not happening in the liver of GMP synthetases850 mutant larvae at this stage. MPO staining was performed as previously described (s4). (G and H) H&E staining of sections through the liver of 7 dpf wild-type (G) and GMP synthetases850 mutant (H) larvae. Hepatic cellular organization appeared to be normal in GMP synthetases850 mutant larvae at 7 dpf.
Supplementary Figure 8. MPA treatment increased the percentage of larvae showing hepatic steatosis in GMP synthetases850 mutant larvae. The percentage of wild-type, GMP synthetases850 mutant and MAP-treated GMP synthetases850 mutant larvae showing hepatic steatosis scored by whole-mount ORO staining at 7 dpf. Animals were treated with15 μg/ml MPA was treated from 3 to 7 dpf. MPA treatment increased the percentage of larvae showing hepatic steatosis in GMP synthetases850 mutant larvae at 7 dpf. **P<0.01; error bars indicate standard deviation.
Supplementary Figure 9. Small molecule inhibitor treatments of earlier stage larvae. The percentage of wild-type larvae treated with DMSO, Rac1 inhibitor or DPI from 3 to 5, from 3 to 6, or from 5 to 7 dpf, showing hepatic steatosis scored by whole-mount ORO staining at 5, 6, or 7 dpf, respectively, are shown. Rac1 inhibitor or DPI treatment from 3 to 5 dpf did not induce hepatic steatosis, while the treatment from 3 to 6 or from 5 to 7 dpf significantly increased the percentage of larvae showing hepatic steatosis. As a control, we also treated wild-type larvae with tunicamicin, and consistent with previous studies(15, 16), tunicamicin-treated larvae significantly increased the percentage of larvae showing ORO staining in the liver at 7 dpf.
Supplementary Figure 10. Small molecule inhibitor treatments on fed-larvae. The percentage of starved wild-type larvae and fed wild-type larvae treated with DMSO, Rac1 inhibitor or DPI from 5 to 7 dpf, showing hepatic steatosis scored by whole-mount ORO staining at 7 dpf. Feeding increased the percentage of larvae showing hepatic steatosis in wild-type larvae, and both Rac1 inhibitor and DPI treatments further increased these percentages. ORO staining experiments with fed wild-type larvae were repeated five times with an average n = 9.8 larvae per experiment (total n = 49 larvae examined and total n = 23showed ORO signal in the liver). With Rac1 inhibitor-treated fed wild-type larvae, the experiments were repeated three times with an average n = 8 larvae per experiment (total n = 24 larvae examined and total n = 20 showed ORO signal in the liver). With DPI-treated fed wild-type larvae, the experiments were repeated three times with an average n = 8 larvae per experiment (total n = 24 larvae examined and total n = 20 showed ORO signal in the liver). *P<0.05, **P<0.01; error bars indicate standard deviation.
Supplementary Figure 11. qPCR analysis of mRNA expression levels in GMP synthetases850 mutant larvae at 6 dpf. The averages of at least three independent experiments are shown. In GMP synthetases850 mutant larvae, tgh mRNA expression level is significantly down-regulated but all other tested mRNA expressions are not significantly changed. ***P<0.001, n.s., not significant; error bars indicate standard deviation.
Acknowledgments
We thank Laura Nagy, Sanjoy Roychowdhury, Jasmine Lau, Takao Sakai, and Thomas McIntyre for critical reading of the manuscript. This work was supported in part by grants from the NIH (R00 DK078138) and CWRU/Cleveland Clinic CTSA (UL1RR024989) and a startup package from the Cleveland Clinic Foundation to T.F.S. This work was also supported by grants from the NIH (R01DK60322) and Packard foundation to D.Y.R.S.
Abbreviations
- NAFLD
non-alcoholic fatty liver disease
- TG
triglyceride
- NASH
nonalcoholic steohepatitis
- TGH
triglyceride hydrolase
- ROS
reactive oxygen species
- GMP
guanosine monophosphate
- GDP
guanosine diphosphate
- GTP
guanosine triphosphate
- dpf
days post-fertilization
- IMP
inosine monophosphate
- XMP
xanthosine monophosphate
- MPA
mycophenolic acid
- PBD
Pak1-binding domain
- DPI
diphenyliodonium
- E600
Diethyl p-nitrophenyl phosphate
References
- 1.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Speliotes EK, Yerges-Armstrong LM, Wu J, Hernaez R, Kim LJ, Palmer CD, Gudnason V, et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7:e1001324. doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmes RS, Wright MW, Laulederkind SJ, Cox LA, Hosokawa M, Imai T, Ishibashi S, et al. Recommended nomenclature for five mammalian carboxylesterase gene families: human, mouse, and rat genes and proteins. Mamm Genome. 2010;21:427–441. doi: 10.1007/s00335-010-9284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Wei E, Quiroga AD, Sun X, Touret N, Lehner R. Altered lipid droplet dynamics in hepatocytes lacking triacylglycerol hydrolase expression. Mol Biol Cell. 2010;21:1991–2000. doi: 10.1091/mbc.E09-05-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei E, Ben Ali Y, Lyon J, Wang H, Nelson R, Dolinsky VW, Dyck JR, et al. Loss of TGH/Ces3 in mice decreases blood lipids, improves glucose tolerance, and increases energy expenditure. Cell Metab. 2010;11:183–193. doi: 10.1016/j.cmet.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Gilham D, Lehner R. Techniques to measure lipase and esterase activity in vitro. Methods. 2005;36:139–147. doi: 10.1016/j.ymeth.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Gilham D, Ho S, Rasouli M, Martres P, Vance DE, Lehner R. Inhibitors of hepatic microsomal triacylglycerol hydrolase decrease very low density lipoprotein secretion. FASEB J. 2003;17:1685–1687. doi: 10.1096/fj.02-0728fje. [DOI] [PubMed] [Google Scholar]
- 8.Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med. 2012;52:59–69. doi: 10.1016/j.freeradbiomed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 10.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 11.Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991;353:668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- 12.Zalkin H, Dixon JE. De novo purine nucleotide biosynthesis. Prog Nucleic Acid Res Mol Biol. 1992;42:259–287. doi: 10.1016/s0079-6603(08)60578-4. [DOI] [PubMed] [Google Scholar]
- 13.Peveri P, Heyworth PG, Curnutte JT. Absolute requirement for GTP in activation of human neutrophil NADPH oxidase in a cell-free system: role of ATP in regenerating GTP. Proc Natl Acad Sci U S A. 1992;89:2494–2498. doi: 10.1073/pnas.89.6.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews RP, Lorent K, Manoral-Mobias R, Huang Y, Gong W, Murray IV, Blair IA, et al. TNFalpha-dependent hepatic steatosis and liver degeneration caused by mutation of zebrafish S-adenosylhomocysteine hydrolase. Development. 2009;136:865–875. doi: 10.1242/dev.027565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cinaroglu A, Gao C, Imrie D, Sadler KC. Activating transcription factor 6 plays protective and pathological roles in steatosis due to endoplasmic reticulum stress in zebrafish. Hepatology. 2011;54:495–508. doi: 10.1002/hep.24396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thakur PC, Stuckenholz C, Rivera MR, Davison JM, Yao JK, Amsterdam A, Sadler KC, et al. Lack of de novo phosphatidylinositol synthesis leads to endoplasmic reticulum stress and hepatic steatosis in cdipt-deficient zebrafish. Hepatology. 2011;54:452–462. doi: 10.1002/hep.24349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hugo SE, Cruz-Garcia L, Karanth S, Anderson RM, Stainier DY, Schlegel A. A monocarboxylate transporter required for hepatocyte secretion of ketone bodies during fasting. Genes Dev. 2012;26:282–293. doi: 10.1101/gad.180968.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Passeri MJ, Cinaroglu A, Gao C, Sadler KC. Hepatic steatosis in response to acute alcohol exposure in zebrafish requires sterol regulatory element binding protein activation. Hepatology. 2009;49:443–452. doi: 10.1002/hep.22667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaub M, Nussbaum J, Verkade H, Ober EA, Stainier DY, Sakaguchi TF. Mutation of zebrafish Snapc4 is associated with loss of the intrahepatic biliary network. Dev Biol. 2012;363:128–137. doi: 10.1016/j.ydbio.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ober EA, Verkade H, Field HA, Stainier DY. Mesodermal Wnt2b signalling positively regulates liver specification. Nature. 2006;442:688–691. doi: 10.1038/nature04888. [DOI] [PubMed] [Google Scholar]
- 21.Greenspan P, Mayer EP, Fowler SD. Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol. 1985;100:965–973. doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu JJ, Stegmann S, Gathy K, Murray R, Laliberte J, Ayscue L, Mitchell BS. Inhibition of T lymphocyte activation in mice heterozygous for loss of the IMPDH II gene. J Clin Invest. 2000;106:599–606. doi: 10.1172/JCI8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long H, Cameron S, Yu L, Rao Y. De novo GMP synthesis is required for axon guidance in Drosophila. Genetics. 2006;172:1633–1642. doi: 10.1534/genetics.105.042911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gummert JF, Barten MJ, Sherwood SW, van Gelder T, Morris RE. Pharmacodynamics of immunosuppression by mycophenolic acid: inhibition of both lymphocyte proliferation and activation correlates with pharmacokinetics. J Pharmacol Exp Ther. 1999;291:1100–1112. [PubMed] [Google Scholar]
- 25.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 26.Bokoch GM, Diebold BA. Current molecular models for NADPH oxidase regulation by Rac GTPase. Blood. 2002;100:2692–2696. doi: 10.1182/blood-2002-04-1149. [DOI] [PubMed] [Google Scholar]
- 27.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng A, Uribe RA, Yieh L, Nuckels R, Gross JM. Zebrafish mutations in gart and paics identify crucial roles for de novo purine synthesis in vertebrate pigmentation and ocular development. Development. 2009;136:2601–2611. doi: 10.1242/dev.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imrie D, Sadler KC. White adipose tissue development in zebrafish is regulated by both developmental time and fish size. Dev Dyn. 2010;239:3013–3023. doi: 10.1002/dvdy.22443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta K, Van Thiel DH, Shah N, Mobarhan S. Nonalcoholic fatty liver disease: pathogenesis and the role of antioxidants. Nutr Rev. 2002;60:289–293. doi: 10.1301/002966402320387224. [DOI] [PubMed] [Google Scholar]
- 31.Lieber CS. S-Adenosyl-L-methionine and alcoholic liver disease in animal models: implications for early intervention in human beings. Alcohol. 2002;27:173–177. doi: 10.1016/s0741-8329(02)00230-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Liver size is reduced in GMP synthetases850 mutant larvae. Brightfield images of wild-type (A) and GMP synthetases850 mutant (B) larvae at 7 dpf are overlaid with Tg(fabp10:GFP-CAAX)lri1 expression (green). GFP expression is shown separately in A′ and B′. (C) EdU incorporation rate in the liver of wild-type and GMP synthetases850 mutant larvae at 4 dpf. The cell proliferation rate, indicated by EdU incorporation, is lower in the liver of GMP synthetases850 mutant larvae at this stage. EdU incorporation was measured as previously described(s1).
Supplementary Figure 2. Positional cloning of the s850 locus. (A) Bulk segregant analyses using markers from the zebrafish genetic map placed the s850 locus on zebrafish chromosome 18 near z14694. We further defined a critical region surrounding the s850 locus, which was flanked by markers 0.05 cM (SCA-KB5) and 0.1 cM (SCA-GA4) from the s850 locus. (B) Sequencing genes in the critical region identified a single nucleotide change (T to A) in the GMP synthetase gene. (C) The s850 mutation changes T589 to A589 of the GMP synthetase gene. (D) The s850 mutation replaces histidine (H189) of GMP synthetase with glutamine (Q189).
Supplementary Figure 3. Electron micrographs of the GMP synthetases850 mutant liver. Electron micrographs of the wild-type (E) and GMP synthetases850 mutant liver at 7 dpf are shown. In GMP synthetases850 mutant larvae, lipid droplets are evident in hepatocytes. l,lipid droplet; n, nucleus.
Supplementary Figure 4. Rac1 expression in the liver. Z-plane confocal image of the dissected liver of wild-type larvae at 7 dpf. (A) Rac1 expression (green) and DAPI staining (white) are visualized. The green channel is shown separately in A′. (B) Mouse IgG1 (geen), used as a negative control in immunofluorescence, and DAPI staining (white) are visualized. The green channel is shown separately in B′. There was no signal detected with mouse IgG1.
Supplementary Figure 5. Tg(fabp10:GFP-DNRac1)lri4 larvae show no morphological defects. (A) Bright-field images of Tg(fabp10:GFP-DNRac1)lri4 larvae at 7 dpf are overlaid with Tg(fabp10:GFP-CAAX)lri1 expression (green). Tg(fabp10:GFP-DNRac1)lri4 larvae are viable and their physical appearance is normal. The liver size of Tg(fabp10:GFP-DNRac1)lri4 larvae is indistinguishable from that of wild-type larvae. (B) Quantification of liver steatosis measured by the percentage of hepatocytes containing Nile Red positive lipid droplets in wild-type sibling, Tg(fabp10:GFP-CAAX)lri1 and hydrogen peroxide-treated Tg(fabp10:GFP-CAAX)lri1 larvae. The percentage of hepatocytes containing lipid droplets is significantly increased in Tg(fabp10:GFP-CAAX)lri1 mutant larvae and is ameliorated by the hydrogen peroxide treatment.
Supplementary Figure 6. The intrahepatic biliary and vascular networks exist in GMP synthetases850 mutant larvae. Wild-type (A) and GMP synthetases850 mutant (B) Tg(Tp1-MmHbb:EGFP)um14 Tg(kdrl:memCherry)s896 larvae visualized for GFP, mCherry, and DAPI expression. Z-plane confocal images of the liver at 7 dpf. GFP and mCherry expressions are shown separately in (A′ and B′) and (A″ and B″), respectively. Ventral views, anterior to the top. Although the liver size is smaller, the intrahepatic biliary and vascular networks, visualized by Tg(Tp1-MmHbb:EGFP)um14 and Tg(kdrl:memCherry)s896 expressions(s2, s3) respectively, appear to be formed in GMP synthetases850 mutant larvae.
Supplementary Figure 7. Neutrophil infiltration to the liver is not observed in GMP synthetases850 mutant larvae. (A–F) Myeloid peroxidase (MPO) staining visualizing neutrophils. Wild-type (A and D), GMP synthetases850 mutant (B and E), and 150 μg/ml lipopolysaccharide (LPS) treated wild-type larvae at 7dpf with MPO-positive neutrophils visualized in dark brown in the livers (A–C) and posterior cardinal vein (D–F; arrows). There were no MPO-positive cells observed in the liver of wild-type and GMP synthetases850 mutant larvae, while MPO-positive cells do infiltrate the liver of LPS-treated larvae (arrowheads in C). These data suggest that inflammation, indicated by neutrophil infiltration, is not happening in the liver of GMP synthetases850 mutant larvae at this stage. MPO staining was performed as previously described (s4). (G and H) H&E staining of sections through the liver of 7 dpf wild-type (G) and GMP synthetases850 mutant (H) larvae. Hepatic cellular organization appeared to be normal in GMP synthetases850 mutant larvae at 7 dpf.
Supplementary Figure 8. MPA treatment increased the percentage of larvae showing hepatic steatosis in GMP synthetases850 mutant larvae. The percentage of wild-type, GMP synthetases850 mutant and MAP-treated GMP synthetases850 mutant larvae showing hepatic steatosis scored by whole-mount ORO staining at 7 dpf. Animals were treated with15 μg/ml MPA was treated from 3 to 7 dpf. MPA treatment increased the percentage of larvae showing hepatic steatosis in GMP synthetases850 mutant larvae at 7 dpf. **P<0.01; error bars indicate standard deviation.
Supplementary Figure 9. Small molecule inhibitor treatments of earlier stage larvae. The percentage of wild-type larvae treated with DMSO, Rac1 inhibitor or DPI from 3 to 5, from 3 to 6, or from 5 to 7 dpf, showing hepatic steatosis scored by whole-mount ORO staining at 5, 6, or 7 dpf, respectively, are shown. Rac1 inhibitor or DPI treatment from 3 to 5 dpf did not induce hepatic steatosis, while the treatment from 3 to 6 or from 5 to 7 dpf significantly increased the percentage of larvae showing hepatic steatosis. As a control, we also treated wild-type larvae with tunicamicin, and consistent with previous studies(15, 16), tunicamicin-treated larvae significantly increased the percentage of larvae showing ORO staining in the liver at 7 dpf.
Supplementary Figure 10. Small molecule inhibitor treatments on fed-larvae. The percentage of starved wild-type larvae and fed wild-type larvae treated with DMSO, Rac1 inhibitor or DPI from 5 to 7 dpf, showing hepatic steatosis scored by whole-mount ORO staining at 7 dpf. Feeding increased the percentage of larvae showing hepatic steatosis in wild-type larvae, and both Rac1 inhibitor and DPI treatments further increased these percentages. ORO staining experiments with fed wild-type larvae were repeated five times with an average n = 9.8 larvae per experiment (total n = 49 larvae examined and total n = 23showed ORO signal in the liver). With Rac1 inhibitor-treated fed wild-type larvae, the experiments were repeated three times with an average n = 8 larvae per experiment (total n = 24 larvae examined and total n = 20 showed ORO signal in the liver). With DPI-treated fed wild-type larvae, the experiments were repeated three times with an average n = 8 larvae per experiment (total n = 24 larvae examined and total n = 20 showed ORO signal in the liver). *P<0.05, **P<0.01; error bars indicate standard deviation.
Supplementary Figure 11. qPCR analysis of mRNA expression levels in GMP synthetases850 mutant larvae at 6 dpf. The averages of at least three independent experiments are shown. In GMP synthetases850 mutant larvae, tgh mRNA expression level is significantly down-regulated but all other tested mRNA expressions are not significantly changed. ***P<0.001, n.s., not significant; error bars indicate standard deviation.